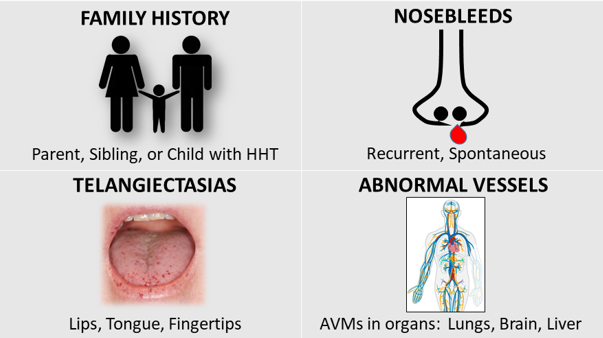
🧵A thread on HHT therapies 🩸
With the publication of the largest powered RCT of a systemic therapy for the 2nd most prevalent inherited bleeding disorder, let’s discuss our systemic therapies for bleeding in hereditary hemorrhagic telangiectasia (#HHT)⤵️
#hemetwitter
1/21
With the publication of the largest powered RCT of a systemic therapy for the 2nd most prevalent inherited bleeding disorder, let’s discuss our systemic therapies for bleeding in hereditary hemorrhagic telangiectasia (#HHT)⤵️
#hemetwitter
1/21
First systemic therapy 👉antifibrinolytics
For mild-mod epistaxis or GIB, tranexamic acid is a mainstay
ATERO is the largest TXA study, randomizing 118 pts in cross-over design, and showing 17% shorter epistaxis duration - a modest but significant effect on epistaxis
4/
For mild-mod epistaxis or GIB, tranexamic acid is a mainstay
ATERO is the largest TXA study, randomizing 118 pts in cross-over design, and showing 17% shorter epistaxis duration - a modest but significant effect on epistaxis
4/
Antifibrinolytic quick notes:
➡️Pill burden creates a real adherence issue (TXA is q8, ε-ACA is q6)
➡️Thromboembolic risk exists theoretically but hasn’t been borne out in trials
➡️ε-ACA likely has similar efficacy but is less well-studied in HHT
sciencedirect.com
5/
➡️Pill burden creates a real adherence issue (TXA is q8, ε-ACA is q6)
➡️Thromboembolic risk exists theoretically but hasn’t been borne out in trials
➡️ε-ACA likely has similar efficacy but is less well-studied in HHT
sciencedirect.com
5/
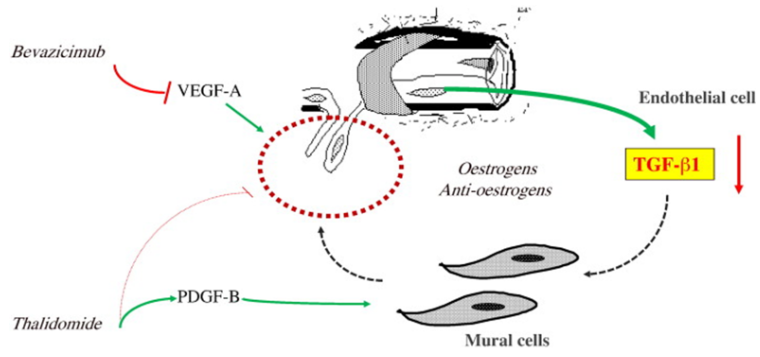
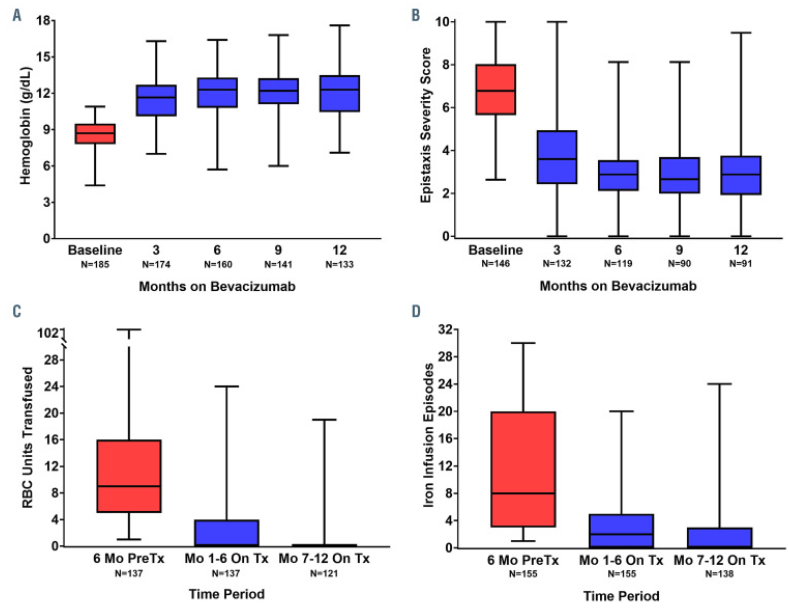
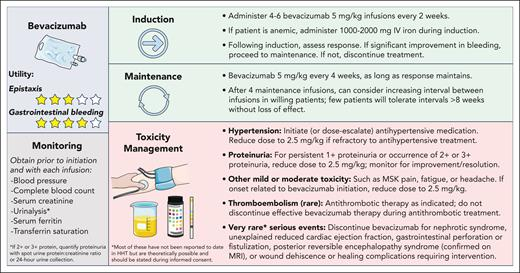
👉Bevacizumab (bev)
Bev is an anti-VEGF mAb that had prevalent off-label use in HHT preceding the multicenter retrospective InHIBIT-BLEED study, which showed improved anemia, ESS, and transfusions in a pre/post analysis
ncbi.nlm.nih.gov
7/
Bev is an anti-VEGF mAb that had prevalent off-label use in HHT preceding the multicenter retrospective InHIBIT-BLEED study, which showed improved anemia, ESS, and transfusions in a pre/post analysis
ncbi.nlm.nih.gov
7/
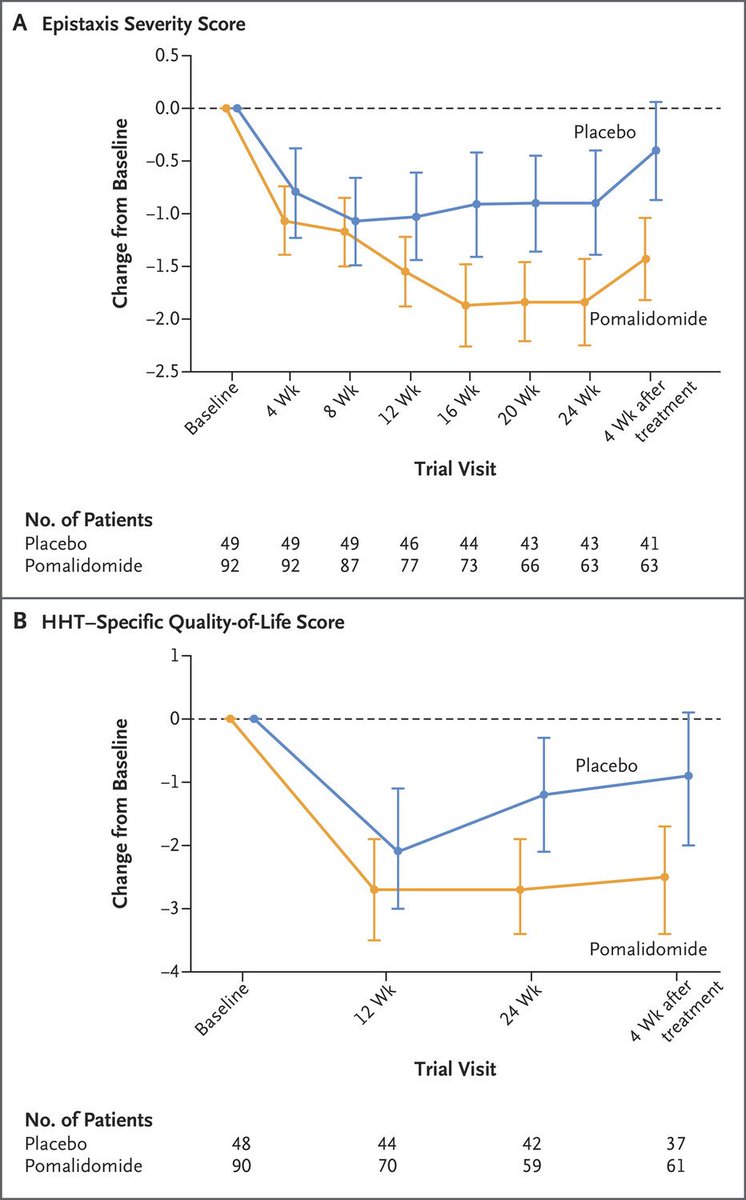
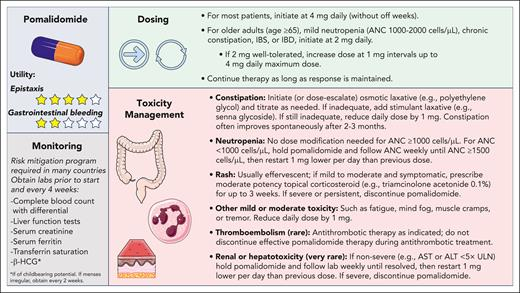
👉Pomalidomide (pom) has antiangiogenic and immunomodulatory effects just like thalidomide, which has shown efficacy in phase 2 study in HHT
PATH-HHT was published in @NEJM this week!
Topline results:
9/
PATH-HHT was published in @NEJM this week!
Topline results:
9/
Cohen's d=0.57, indicating medium effect size
ESS diff becomes apparent ~12 wks after pom initiation and even maintains at 1 month post-treatment, suggestive of persistent effect, perhaps via improved vascular integrity, continued VEGF or bFGF suppression, or other MoA...?
11/
ESS diff becomes apparent ~12 wks after pom initiation and even maintains at 1 month post-treatment, suggestive of persistent effect, perhaps via improved vascular integrity, continued VEGF or bFGF suppression, or other MoA...?
11/
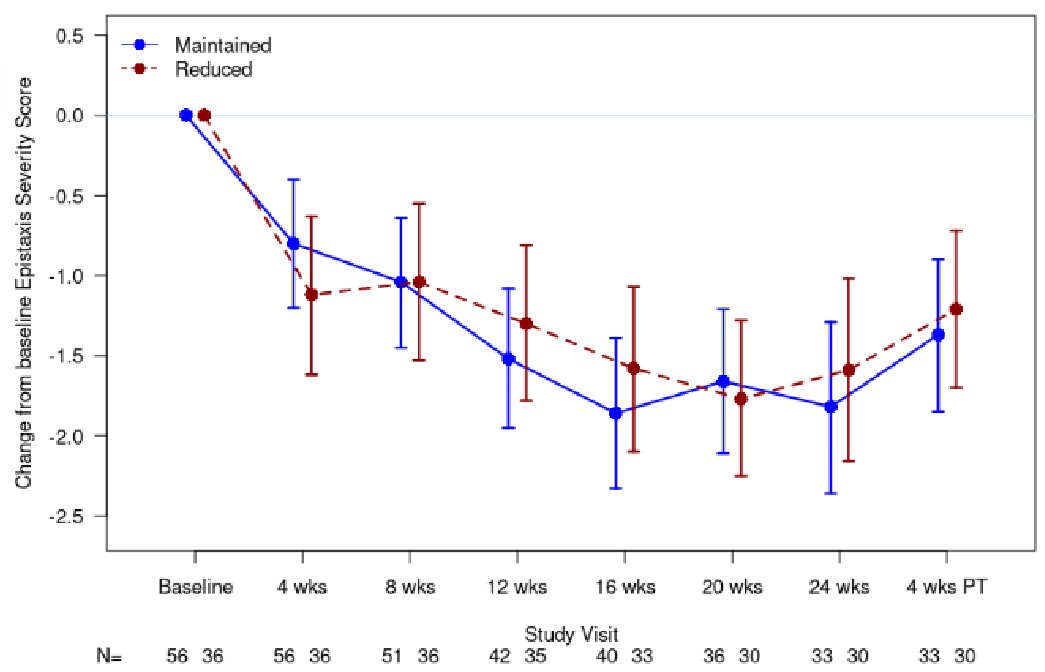
There’s a notable discontinuation rate for pom, which isn’t unexpected but seems a bit higher than in myeloma...maybe that’s due to continuous dosing vs MM 3 wk on / 1 wk off dosing ? Open to other ideas here.
Maybe we just have higher thresholds for holding cancer therapy?
12/
Maybe we just have higher thresholds for holding cancer therapy?
12/
Eventually, studies will compare bev and pom, hopefully in randomized design, but in the meantime, comorbidities and preferences are salient to shared decision-making
Expert opinion suggests bev is preferred for GIB vs pom for epistaxis
How may comorbidities sway you?
15/
Expert opinion suggests bev is preferred for GIB vs pom for epistaxis
How may comorbidities sway you?
15/
CHF, uncontrolled HTN, osteoporosis requiring antiresorptive tx, and nephrotic syndromes may favor pom over bev
Pre-existing neutropenia or thrombocytopenia, EtOH use, constipation, IBS, or IBD may favor bev over pom
16/
Pre-existing neutropenia or thrombocytopenia, EtOH use, constipation, IBS, or IBD may favor bev over pom
16/
There are no approved therapies for HHT but a few others of note:
👉Pazopanib - antiangiogenic TKI, esp targeting VEGF-R
👉Octreotide / lanreotide - somatostatin analogs with observational data on ⬇️GIB
👉Thalidomide - IMiD with worse safety profile than pom - bye!👋
17/
👉Pazopanib - antiangiogenic TKI, esp targeting VEGF-R
👉Octreotide / lanreotide - somatostatin analogs with observational data on ⬇️GIB
👉Thalidomide - IMiD with worse safety profile than pom - bye!👋
17/
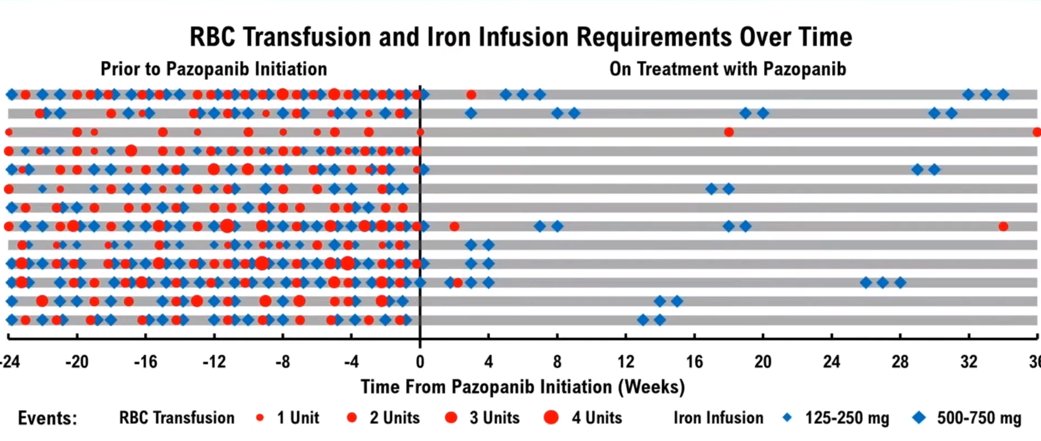
Pazopanib is currently in a placebo-controlled phase 2/3 trial (NCT03850964) at very low dose relative to dosing across malignancies
Prior observational data suggests its utility in reducing transfusion dependence
ncbi.nlm.nih.gov
18/
Prior observational data suggests its utility in reducing transfusion dependence
ncbi.nlm.nih.gov
18/
Finally, I’ll leave estrogens/SERMs aside for a later deep dive as data is mixed across small studies and there’s a notable thrombotic risk
Doxycycline is often considered as well but has negative results across a couple trials
19/
Doxycycline is often considered as well but has negative results across a couple trials
19/
The ability for the trial to recruit enough participants in a condition as rare as HHT speaks to the collaborative nature of the HHT Centers of Excellence model and to patient organizations like @CureHHT that raise awareness and fund research
#raredisease
20/
#raredisease
20/
A round of applause for the authors on conducting a randomized and well-powered study for a systemic agent in a rare disease - a labor of love surely. Such data doesn’t exist for bevacizumab or pazopanib yet, but soon it may!
A great recent ref: ashpublications.org
end!
A great recent ref: ashpublications.org
end!
Loading suggestions...