📍Living kidney donation increases access to transplantation for ESKD patients and offers better outcomes compared to deceased donations.
📍Evaluating LKD candidates involves thorough medical, surgical, psychosocial, and financial assessments to ensure a safe donation process.
📍Approximately 32,000 LDKTs are performed annually worldwide, accounting for nearly 40% of all kidney transplants.
📍 LKDs have outcomes similar to or better than healthy controls.
📍 Comprehensive evaluations minimize perioperative and long-term risks.
📍Evaluating LKD candidates involves thorough medical, surgical, psychosocial, and financial assessments to ensure a safe donation process.
📍Approximately 32,000 LDKTs are performed annually worldwide, accounting for nearly 40% of all kidney transplants.
📍 LKDs have outcomes similar to or better than healthy controls.
📍 Comprehensive evaluations minimize perioperative and long-term risks.
Hypertension (HTN)
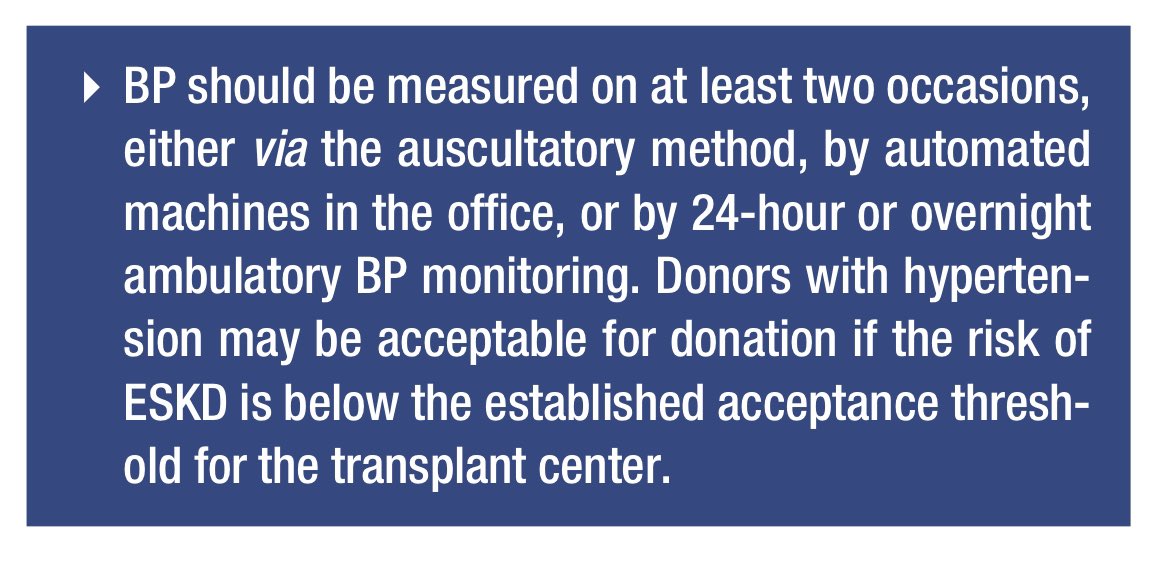
📍BP assessment is crucial for LKD candidates.
📍OPTN policy requires BP measurements on at least 2 occasions either via auscultatory, automated machines, or ambulatory BP monitoring (ABPM).
📍ABPM is the gold standard but less available.
📍New guidelines from the ACC/AHA define hypertension as BP ≥130/80 mm Hg, a change from the previous <140/90 mm Hg threshold.
📍This impacts the evaluation of LKD candidates
📍Studies show HTN prevalence among LKD candidates is higher with the new threshold (34%) vs the old one (16%).
📍KDIGO guidelines for LKD evaluation still use older JNC-7 thresholds <140/90 mm, but the new ACC/AHA thresholds are controversial.
📍Explain to LKD candidates that HTN definitions may differ among clinicians, impacting their diagnoses.
📍Merzkani et al found Predonation HTN (BP ≥130/80 mm Hg) doesn't ↑post-donation risks for ↓ eGFR or proteinuria.
📍However, a baseline BP ≥140/90 mm Hg does ↑ the risk of proteinuria.
📍National data shows that LKDs with predonation HTN (BP ≥140/90 mm Hg) have a ↑ 15-year risk of ESKD (0.8%) compared to those without HTN (0.2%).
📍Studies indicate that LKDs have a higher risk of developing HTN post-donation compared to non-donors
📍BP assessment is crucial for LKD candidates.
📍OPTN policy requires BP measurements on at least 2 occasions either via auscultatory, automated machines, or ambulatory BP monitoring (ABPM).
📍ABPM is the gold standard but less available.
📍New guidelines from the ACC/AHA define hypertension as BP ≥130/80 mm Hg, a change from the previous <140/90 mm Hg threshold.
📍This impacts the evaluation of LKD candidates
📍Studies show HTN prevalence among LKD candidates is higher with the new threshold (34%) vs the old one (16%).
📍KDIGO guidelines for LKD evaluation still use older JNC-7 thresholds <140/90 mm, but the new ACC/AHA thresholds are controversial.
📍Explain to LKD candidates that HTN definitions may differ among clinicians, impacting their diagnoses.
📍Merzkani et al found Predonation HTN (BP ≥130/80 mm Hg) doesn't ↑post-donation risks for ↓ eGFR or proteinuria.
📍However, a baseline BP ≥140/90 mm Hg does ↑ the risk of proteinuria.
📍National data shows that LKDs with predonation HTN (BP ≥140/90 mm Hg) have a ↑ 15-year risk of ESKD (0.8%) compared to those without HTN (0.2%).
📍Studies indicate that LKDs have a higher risk of developing HTN post-donation compared to non-donors
(Obesity)
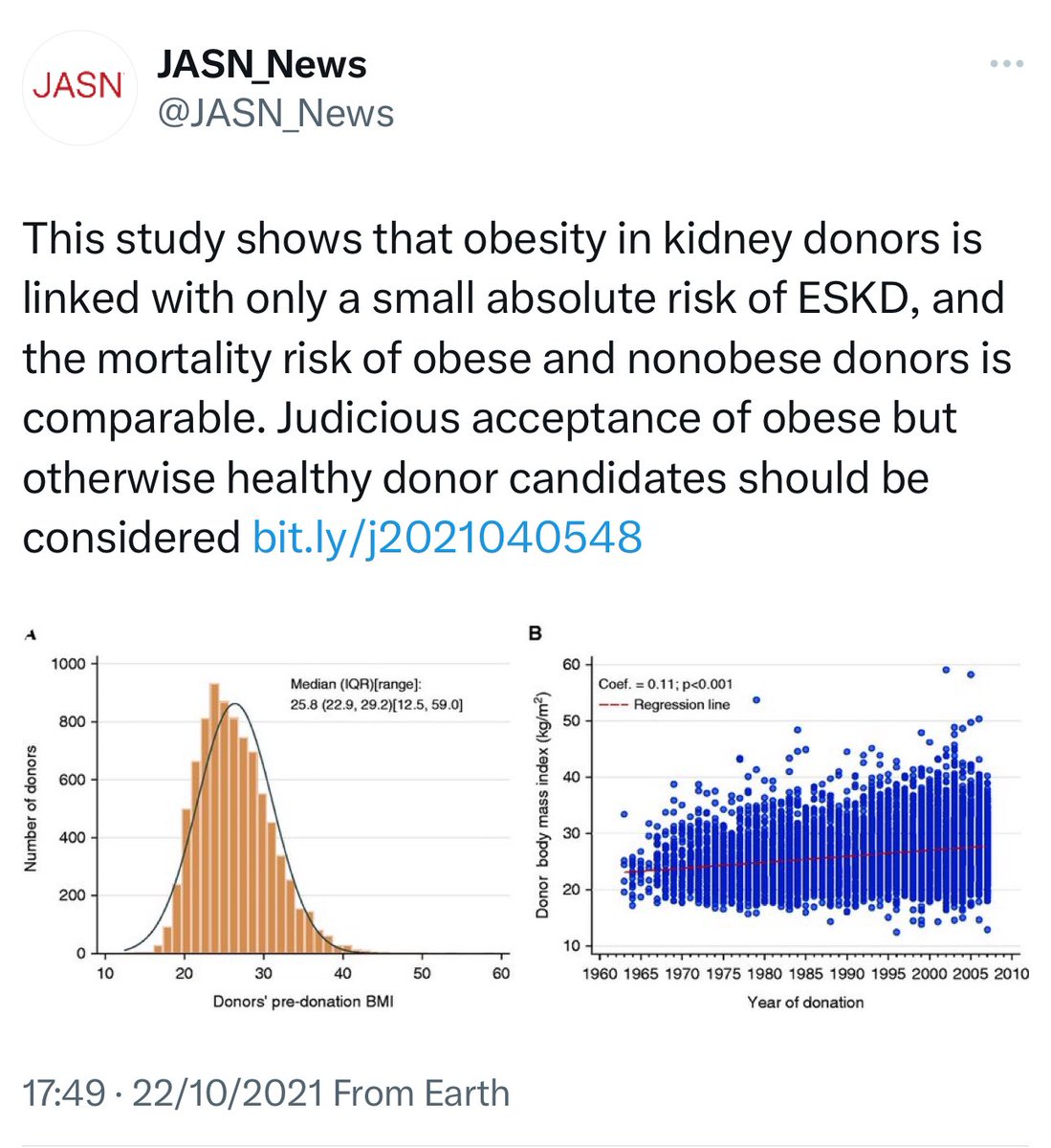
📍In the U.S, as obesity rates ↑, more LKD candidates are overweight or mildly obese.
📍The effect of obesity on post-donation outcomes remains unclear.
📍A meta-analysis suggests only a modest ↑ in ESKD risk for individuals with BMI >30 kg/m² over 4-16 years.
📍In LKDs, nephrectomy (↓ kidney mass) may ↑ hyperfiltration stress from obesity, impacting long-term kidney health.
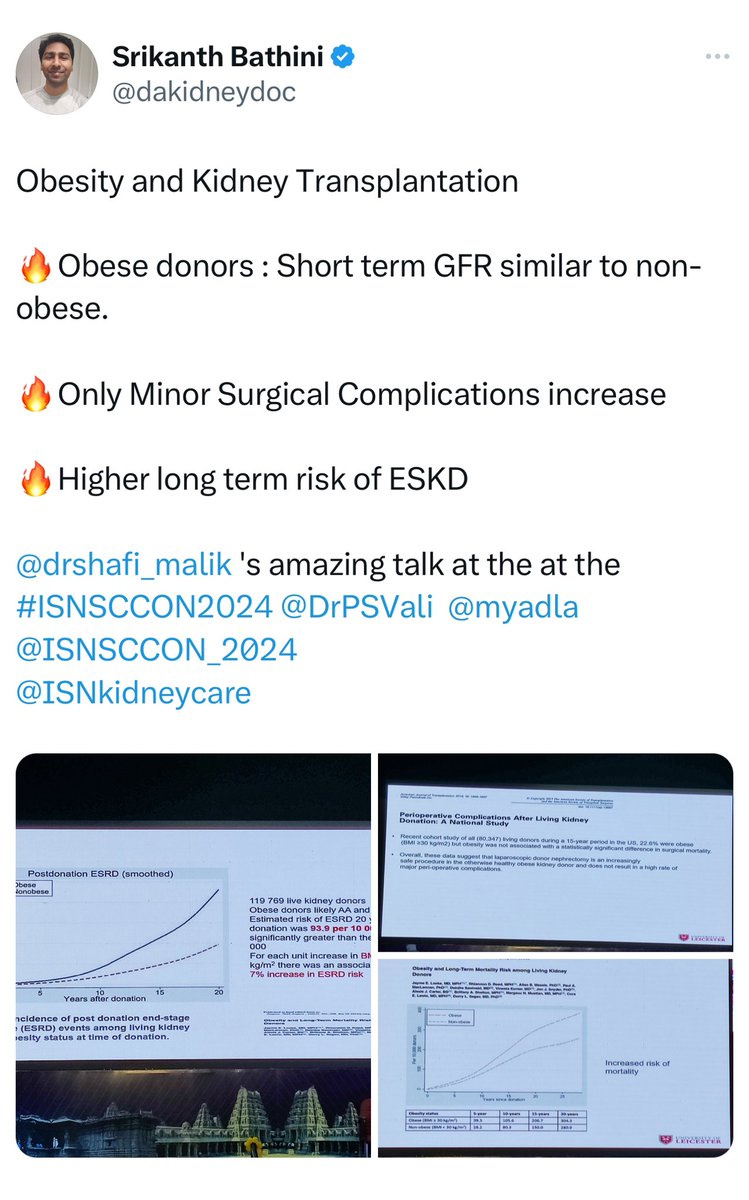
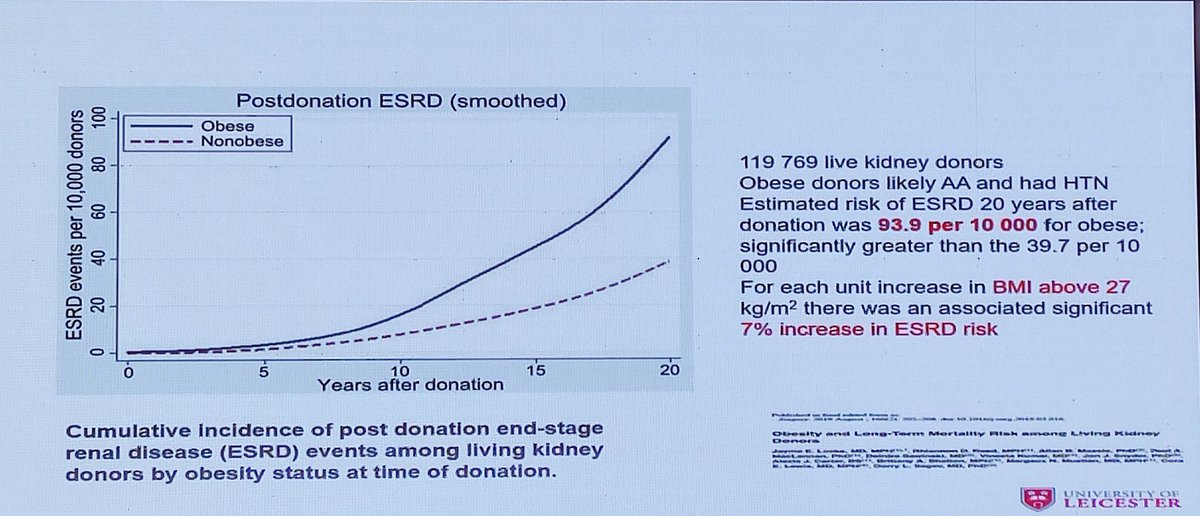
📍Obese LKDs (BMI >30 kg/m²) have a higher 20-year risk of ESKD (93.9 per 10,000) vs. non-obese donors (39.7 per 10,000).
📍Mortality risk is also 30% higher in obese donors.
📍Obesity ↑ mortality risk in both the general population and LKDs.
📍Pek et al. used cross-sectional imaging to identify LKDs with visceral obesity (≥100 cm² at the umbilicus) and found it was linked to a larger ↓ in postdonation eGFR.
📍Obesity ↑ risks for DM and HTN, which can lead to CKD over time.
📍Obese LKDs are 3x more likely to develop DM and 75% more likely to develop HTN post-donation.
📍Overweight and obese LKDs are 2-4x more likely to require antidiabetic meds within 9 years post-donation.
📍The 2017 KDIGO guideline recommends individualizing decisions for LKD candidates with BMI >30 kg/m² based on their health profile and the program's risk threshold.
📍In the U.S, as obesity rates ↑, more LKD candidates are overweight or mildly obese.
📍The effect of obesity on post-donation outcomes remains unclear.
📍A meta-analysis suggests only a modest ↑ in ESKD risk for individuals with BMI >30 kg/m² over 4-16 years.
📍In LKDs, nephrectomy (↓ kidney mass) may ↑ hyperfiltration stress from obesity, impacting long-term kidney health.
📍Obese LKDs (BMI >30 kg/m²) have a higher 20-year risk of ESKD (93.9 per 10,000) vs. non-obese donors (39.7 per 10,000).
📍Mortality risk is also 30% higher in obese donors.
📍Obesity ↑ mortality risk in both the general population and LKDs.
📍Pek et al. used cross-sectional imaging to identify LKDs with visceral obesity (≥100 cm² at the umbilicus) and found it was linked to a larger ↓ in postdonation eGFR.
📍Obesity ↑ risks for DM and HTN, which can lead to CKD over time.
📍Obese LKDs are 3x more likely to develop DM and 75% more likely to develop HTN post-donation.
📍Overweight and obese LKDs are 2-4x more likely to require antidiabetic meds within 9 years post-donation.
📍The 2017 KDIGO guideline recommends individualizing decisions for LKD candidates with BMI >30 kg/m² based on their health profile and the program's risk threshold.
(Assessment of GFR)
📍Assessing kidney function is crucial for LKD candidates.
📍OPTN requires CrCl or measured GFR with exogenous markers (iothalamate/iohexol).
📍Measured GFR is more accurate but costly and time-consuming.
📍No single method is standardized; all have measurement errors.
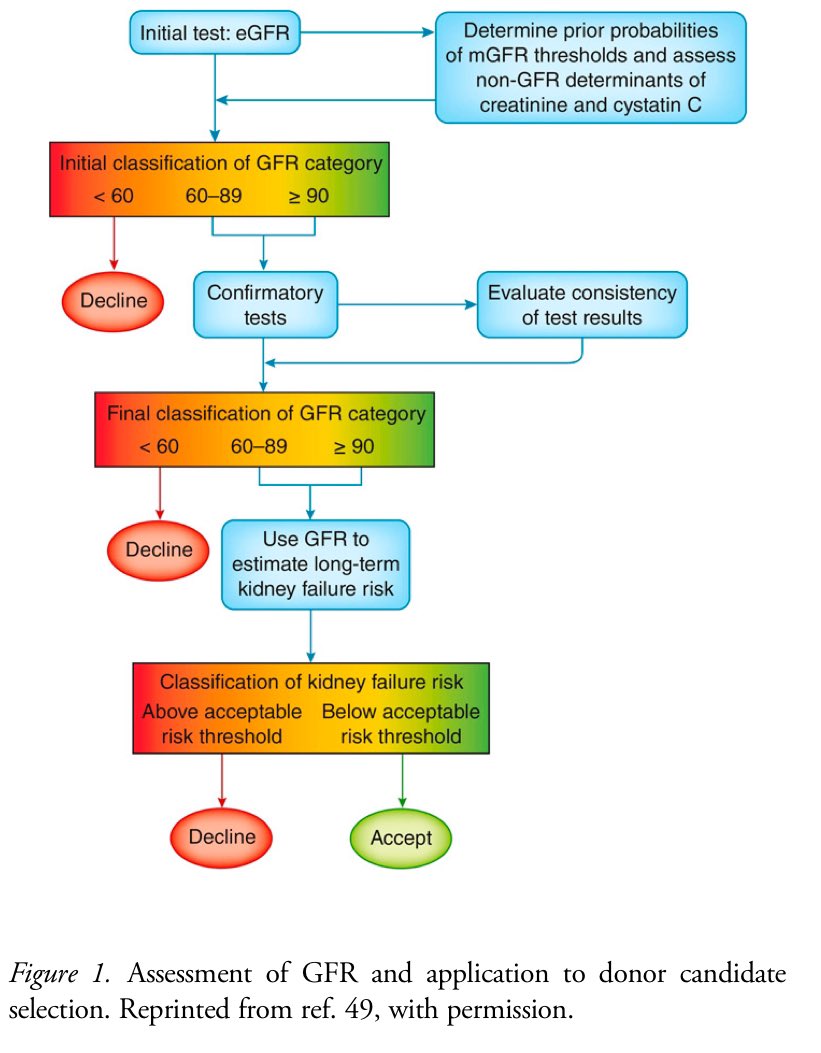
📍2017 KDIGO guideline suggests initial screening with creatinine-based eGFR, followed by confirmatory testing with : measured GFR using an exogenous marker, CrCl, eGFR with combinations of serum creatinine and cystatin C or repeat eGFR.
📍Transplant centers may use eGFR before the LKD visit.
📍Web calculators estimate GFR probability using creatinine or cystatin C. (ckdepi.org).
📍High probability of low GFR (<60 ml/min/1.73 m²) can exclude candidates early.
📍Intermediate probability or U.S. candidates need confirmatory testing with measured GFR or CrCl.
📍Measured GFR or CrCl can early exclude ineligible candidates; confirmatory tests for others.
📍Race coefficients in GFR equations are controversial.
📍Most staff find race-based calculators inappropriate.
📍NKF/ASN suggests a race-neutral CKD-EPI equation.
📍Race-neutral eGFR might exclude some Black candidates near thresholds.
📍OPTN requires confirmatory measured GFR or CrCl to avoid disparities.
📍Studies show : CrCl often overestimates, eGFR underestimates true GFR.
📍Combining CrCl and eGFR is better than CrCl alone.
📍Cortical Volume Ratio defined as the ratio of the cortical volume of the non donated kidney to the total cortical volume from CT angiogram, may predict post-donation eGFR more accurately but needs special software/training.
👇Here's a flowchart for assessing GFR and selecting kidney donor candidates
📍Assessing kidney function is crucial for LKD candidates.
📍OPTN requires CrCl or measured GFR with exogenous markers (iothalamate/iohexol).
📍Measured GFR is more accurate but costly and time-consuming.
📍No single method is standardized; all have measurement errors.
📍2017 KDIGO guideline suggests initial screening with creatinine-based eGFR, followed by confirmatory testing with : measured GFR using an exogenous marker, CrCl, eGFR with combinations of serum creatinine and cystatin C or repeat eGFR.
📍Transplant centers may use eGFR before the LKD visit.
📍Web calculators estimate GFR probability using creatinine or cystatin C. (ckdepi.org).
📍High probability of low GFR (<60 ml/min/1.73 m²) can exclude candidates early.
📍Intermediate probability or U.S. candidates need confirmatory testing with measured GFR or CrCl.
📍Measured GFR or CrCl can early exclude ineligible candidates; confirmatory tests for others.
📍Race coefficients in GFR equations are controversial.
📍Most staff find race-based calculators inappropriate.
📍NKF/ASN suggests a race-neutral CKD-EPI equation.
📍Race-neutral eGFR might exclude some Black candidates near thresholds.
📍OPTN requires confirmatory measured GFR or CrCl to avoid disparities.
📍Studies show : CrCl often overestimates, eGFR underestimates true GFR.
📍Combining CrCl and eGFR is better than CrCl alone.
📍Cortical Volume Ratio defined as the ratio of the cortical volume of the non donated kidney to the total cortical volume from CT angiogram, may predict post-donation eGFR more accurately but needs special software/training.
👇Here's a flowchart for assessing GFR and selecting kidney donor candidates
(Nephrolithiasis)
📍Nephrolithiasis is not uncommon in LKD candidates.
📍Increased imaging sensitivity has led to more incidental detections of small asymptomatic stones.
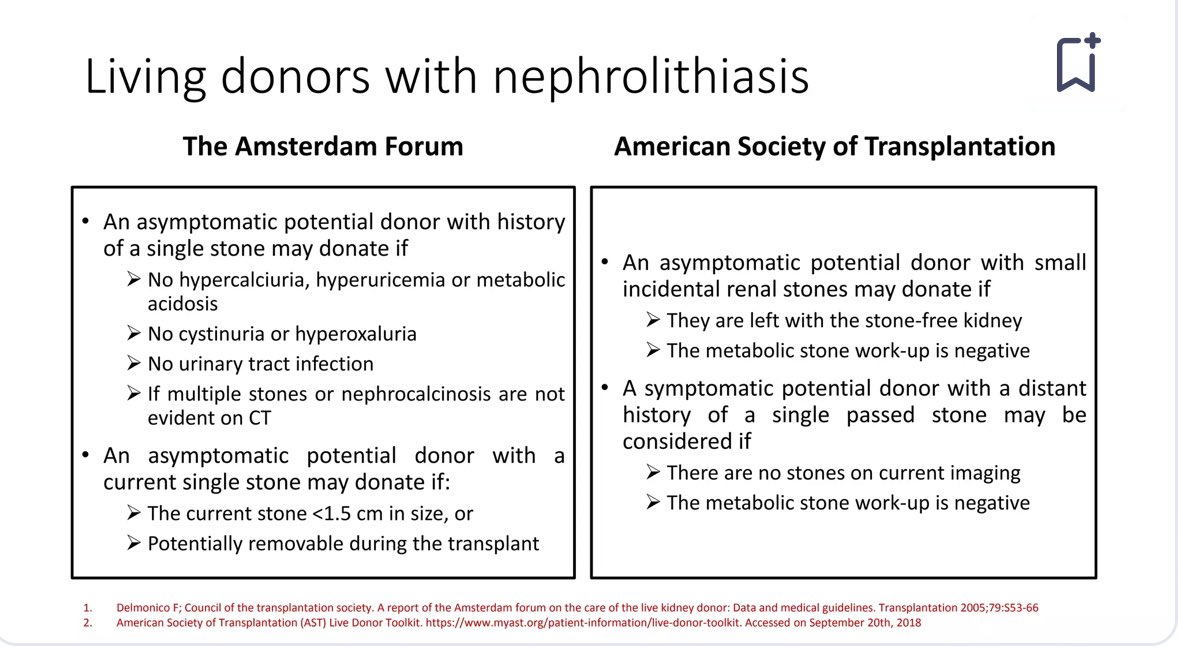
📍2017 KDIGO recommends : Evaluate all donor candidates with kidney stone history for underlying causes.
📍Acceptance depends on recurrence risk and candidate's understanding of consequences.
📍Major risk for LKDs with kidney stone: post-nephrectomy recurrence.
📍This can lead to obstruction, infections, or structural damage to the remaining kidney.
📍Data on stones outcomes in LKDs is limited
📍General population data shows a recurrence rate of 15/100 person-years for calcium-stone formers.
📍Post hoc RELIVE study: Compared LKDs with and without kidney stones.
📍No significant link found between history of stones and ↓ kidney function, HTN, or proteinuria at 17-year follow-up.
📍RELIVE study limitations: no data on 24-hr urine profiles, stone types, or risk-reduction
📍Despite this, findings help inform consent for LKD candidates with stones .
👇Here's a Comparison of recommendations for living donors with nephrolithiasis
📍Nephrolithiasis is not uncommon in LKD candidates.
📍Increased imaging sensitivity has led to more incidental detections of small asymptomatic stones.
📍2017 KDIGO recommends : Evaluate all donor candidates with kidney stone history for underlying causes.
📍Acceptance depends on recurrence risk and candidate's understanding of consequences.
📍Major risk for LKDs with kidney stone: post-nephrectomy recurrence.
📍This can lead to obstruction, infections, or structural damage to the remaining kidney.
📍Data on stones outcomes in LKDs is limited
📍General population data shows a recurrence rate of 15/100 person-years for calcium-stone formers.
📍Post hoc RELIVE study: Compared LKDs with and without kidney stones.
📍No significant link found between history of stones and ↓ kidney function, HTN, or proteinuria at 17-year follow-up.
📍RELIVE study limitations: no data on 24-hr urine profiles, stone types, or risk-reduction
📍Despite this, findings help inform consent for LKD candidates with stones .
👇Here's a Comparison of recommendations for living donors with nephrolithiasis
(Genetic Kidney Disease) 🧬
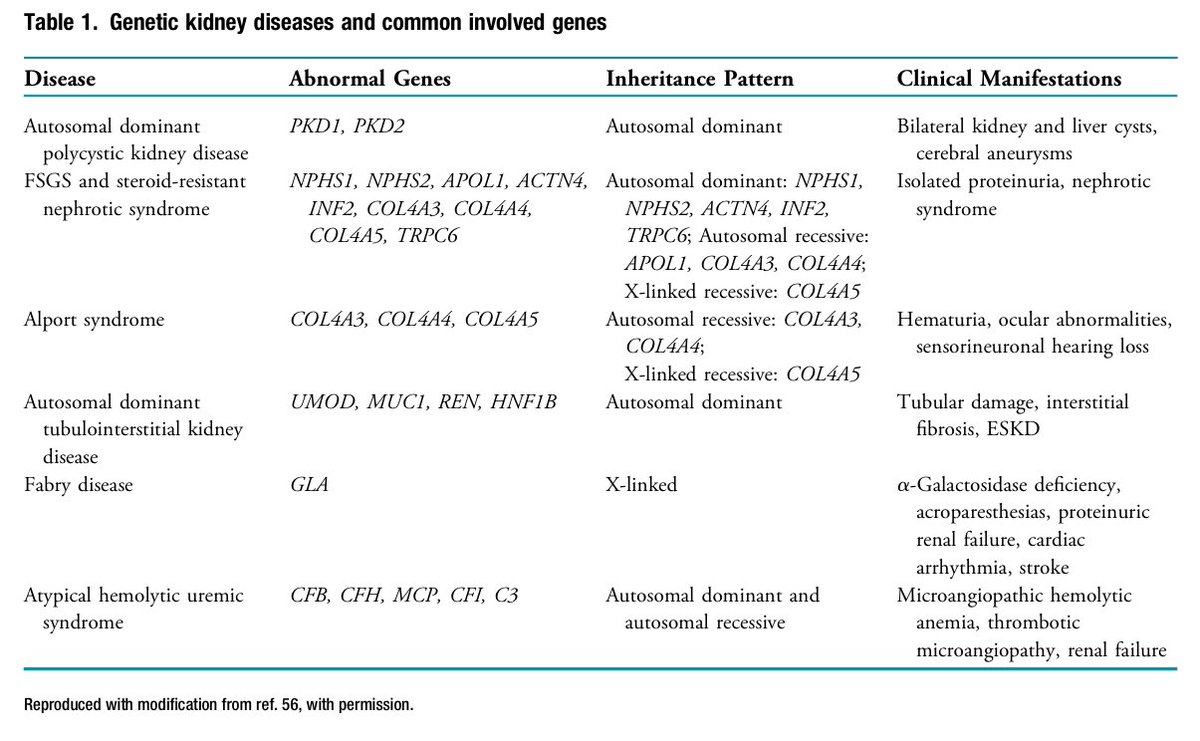
📍2017 KDIGO recommends Evaluate LKD candidates for inherited kidney disease, especially with a family hx of kidney failure or unknown recipient cause.
📍US OPTN recommends Programs must develop protocols for assessing inherited kidney diseases based on family hx.
📍Review family hx to identify conditions.
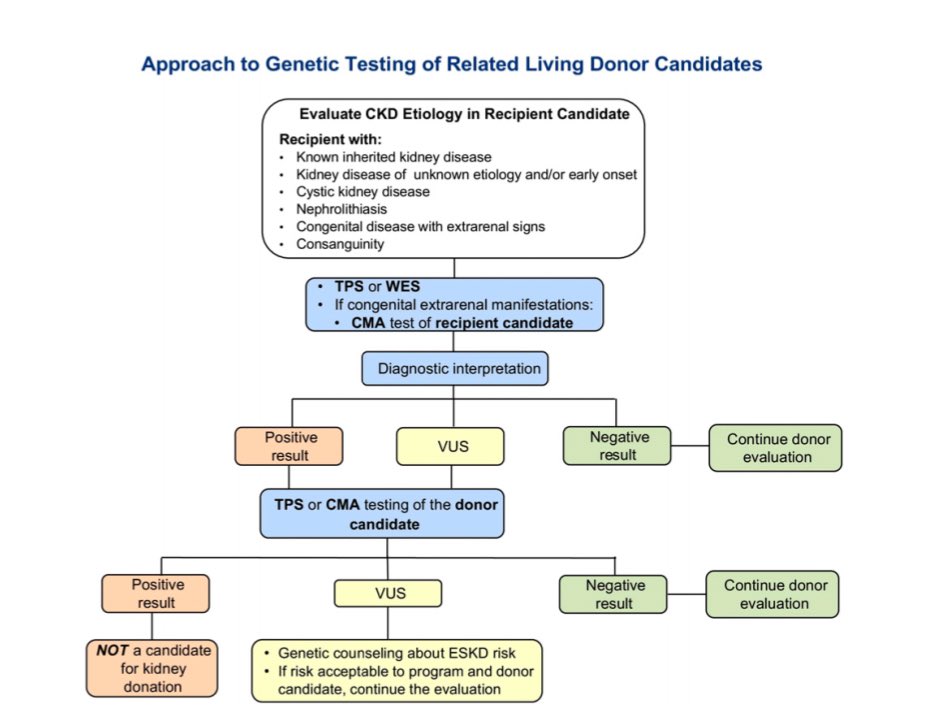
📍Genetic testing, with counseling, helps assess candidacy and inform decisions.
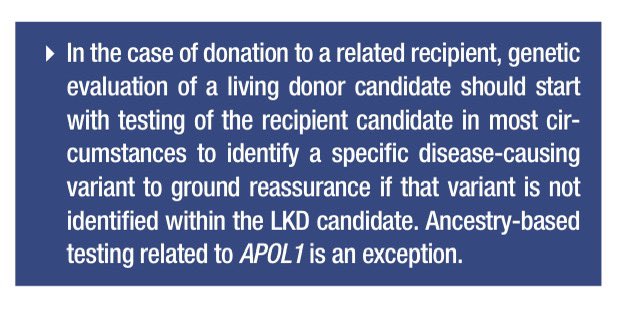
📍Genetic renal panels have made testing more feasible. Start with the recipient to identify disease-causing variants.
📍Genetic testing can assess single or multiple genes via next-gen sequencing.
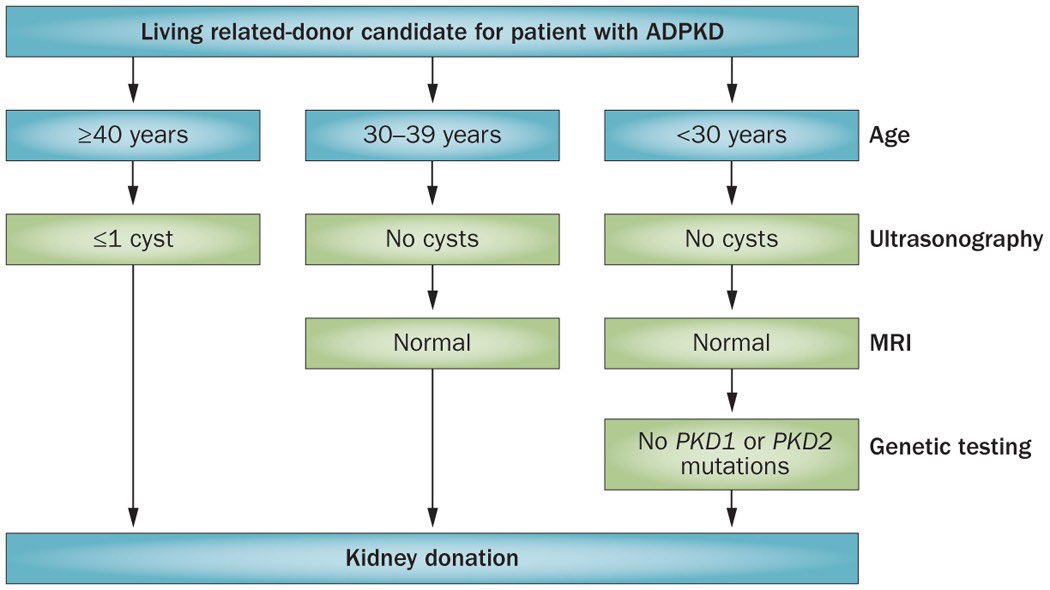
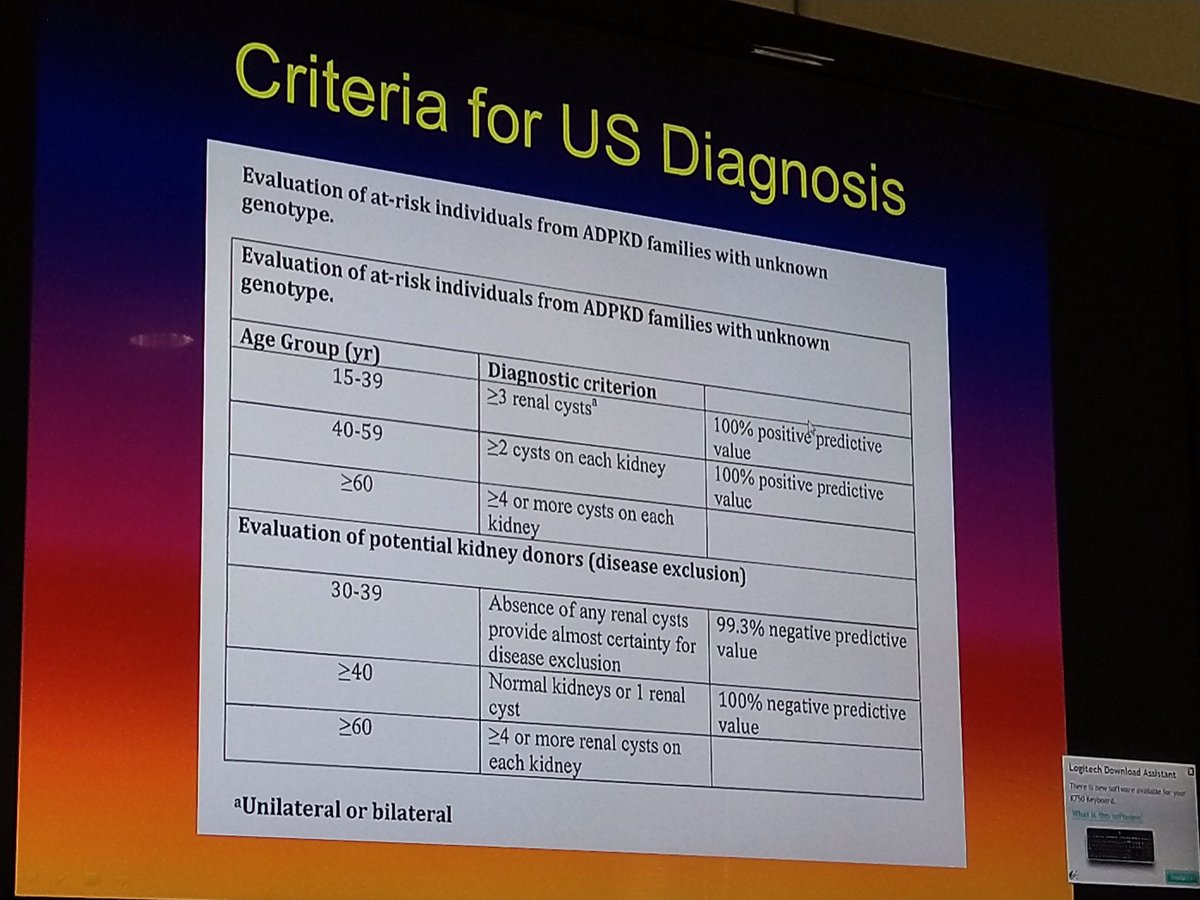
📍Guidelines exist for evaluating LKDs with ADPKD.
👇Criteria for kidney donation by family members of patients with ADPKD
📍2017 KDIGO recommends Evaluate LKD candidates for inherited kidney disease, especially with a family hx of kidney failure or unknown recipient cause.
📍US OPTN recommends Programs must develop protocols for assessing inherited kidney diseases based on family hx.
📍Review family hx to identify conditions.
📍Genetic testing, with counseling, helps assess candidacy and inform decisions.
📍Genetic renal panels have made testing more feasible. Start with the recipient to identify disease-causing variants.
📍Genetic testing can assess single or multiple genes via next-gen sequencing.
📍Guidelines exist for evaluating LKDs with ADPKD.
👇Criteria for kidney donation by family members of patients with ADPKD
(Genetic Kidney Disease) continues... 🧬
📍LKDs can be screened for rare forms of ADTKD, hereditary FSGS, atypical HUS, Alport syndrome, and Fabry disease.
📍Among Black individuals, APOL1 variants (G1 or G2) ↑ kidney failure risk.
📍Studies showed Black individuals with two APOL1 variants have ↑ risk of reduced eGFR and faster decline post-donation.
📍NIH APOLLO and LETO studies aim to define APOL1 genotyping's role in kidney transplantation and LKD, focusing on long-term outcomes for Black donors and recipients
📍2017 KDIGO recommends Inform Black donor candidates about the ↑ risk of kidney failure with two APOL1 variants.
📍2021 panel: APOL1 testing should involve shared decision-making. Proceed with donation only if ESKD risk is acceptable.
📍LKDs can be screened for rare forms of ADTKD, hereditary FSGS, atypical HUS, Alport syndrome, and Fabry disease.
📍Among Black individuals, APOL1 variants (G1 or G2) ↑ kidney failure risk.
📍Studies showed Black individuals with two APOL1 variants have ↑ risk of reduced eGFR and faster decline post-donation.
📍NIH APOLLO and LETO studies aim to define APOL1 genotyping's role in kidney transplantation and LKD, focusing on long-term outcomes for Black donors and recipients
📍2017 KDIGO recommends Inform Black donor candidates about the ↑ risk of kidney failure with two APOL1 variants.
📍2021 panel: APOL1 testing should involve shared decision-making. Proceed with donation only if ESKD risk is acceptable.
(Postdonation ESKD)
📍Postdonation ESKD is a rare outcome, occurring decades after donor nephrectomy.
📍This rarity makes it hard to study at a population level.
📍The risk of postdonation ESKD is similar to the general population's risk, according to previous studies.
📍In 2014, 2 major studies compared postdonation ESKD risk with healthy, non-donor controls.
➖🔹 Mjøen et al. studied 1901 kidney donors in 🇳🇴 who donated between 1963 and 2007. The median follow-up was 15.1 years.
📍Of the 1901 donors, 9 (0.47%) developed ESKD with a median time of 18.7 years from donation to ESKD.
📍The control group in Mjøen et al.'s study included 32,621 non-donors, with a median follow-up of 24.9 years.
📍In the control group, 22 (0.07%) developed ESKD, making the risk 11-fold higher in kidney donors compared to non-donors.
➖🔹Muzaale et al. studied 96,217 🇺🇸 kidney donors who donated between 1994 and 2011. The median follow-up was 7.6 years.
📍Of the 96,217 donors, 99 (0.10%) developed ESKD with a mean time of 8.6 years from donation to ESKD.
📍The control group in Muzaale et al.'s study included 20,024 non-donors from the Third National Health and Nutrition Exam Survey.
📍In Muzaale et al.'s control group, 36 (0.04%) developed ESKD, with an estimated 15-year cumulative incidence of 30.8 vs. 3.9 per 10,000 people.
📍The risk of ESKD was 8-fold higher in kidney donors compared to healthy, non-donor controls according to Muzaale et al.
📍Certain subgroups of donors have a higher risk of ESKD: older donors, males, Black, and those related to their recipient.
📍Both studies have limitations, but they suggest the absolute increase in 15-year ESKD risk post-donation is <1%.
📍Postdonation ESKD is a rare outcome, occurring decades after donor nephrectomy.
📍This rarity makes it hard to study at a population level.
📍The risk of postdonation ESKD is similar to the general population's risk, according to previous studies.
📍In 2014, 2 major studies compared postdonation ESKD risk with healthy, non-donor controls.
➖🔹 Mjøen et al. studied 1901 kidney donors in 🇳🇴 who donated between 1963 and 2007. The median follow-up was 15.1 years.
📍Of the 1901 donors, 9 (0.47%) developed ESKD with a median time of 18.7 years from donation to ESKD.
📍The control group in Mjøen et al.'s study included 32,621 non-donors, with a median follow-up of 24.9 years.
📍In the control group, 22 (0.07%) developed ESKD, making the risk 11-fold higher in kidney donors compared to non-donors.
➖🔹Muzaale et al. studied 96,217 🇺🇸 kidney donors who donated between 1994 and 2011. The median follow-up was 7.6 years.
📍Of the 96,217 donors, 99 (0.10%) developed ESKD with a mean time of 8.6 years from donation to ESKD.
📍The control group in Muzaale et al.'s study included 20,024 non-donors from the Third National Health and Nutrition Exam Survey.
📍In Muzaale et al.'s control group, 36 (0.04%) developed ESKD, with an estimated 15-year cumulative incidence of 30.8 vs. 3.9 per 10,000 people.
📍The risk of ESKD was 8-fold higher in kidney donors compared to healthy, non-donor controls according to Muzaale et al.
📍Certain subgroups of donors have a higher risk of ESKD: older donors, males, Black, and those related to their recipient.
📍Both studies have limitations, but they suggest the absolute increase in 15-year ESKD risk post-donation is <1%.
Postdonation Cardiovascular Disease
(CVD)
📍CV events are a leading cause of death among LKDs, accounting for 30-40% of all deaths.
📍A study by Garg et al. of 2,028 LKDs from Ontario (1992-2009) found LKDs had a lower risk of death and major CV events compared to 20,280 healthy nondonor controls over a median follow-up of 6.5 years.
📍The study reported 2.8 vs 4.1 events per 1,000 person-years (HR, 0.66; 95% CI, 0.48 to 0.90) for death and major CV events.
📍Death-censored major CV event risk was similar between LKDs and nondonors.
📍Similar findings in a US cohort of older LKDs (≥55 years) with a median follow-up of 7.8 years showed no significant difference in major CV event risk compared to nondonors.
📍Mjøen et al. found a 40% higher risk of CV death among LKDs compared to healthy controls over a 15.1-year follow-up.
📍Of 224 deaths among 1,901 LKDs, 68 (30.4%) were due to CVD.
📍A follow-up study found a 64% higher risk of diagnosed IHD in LKDs compared to nondonors over a mean follow-up of > 10 years (adjusted OR, 1.64; 95% CI, 1.10 to 2.43).
📍↓ kidney function may be a risk factor for postdonation CV events and death, similar to patterns observed in the general population.
📍Currently, No evidence that predonation cardiac testing or risk factor modification beyond general guidelines reduces long-term CV risk for LKDs

(CVD)
📍CV events are a leading cause of death among LKDs, accounting for 30-40% of all deaths.
📍A study by Garg et al. of 2,028 LKDs from Ontario (1992-2009) found LKDs had a lower risk of death and major CV events compared to 20,280 healthy nondonor controls over a median follow-up of 6.5 years.
📍The study reported 2.8 vs 4.1 events per 1,000 person-years (HR, 0.66; 95% CI, 0.48 to 0.90) for death and major CV events.
📍Death-censored major CV event risk was similar between LKDs and nondonors.
📍Similar findings in a US cohort of older LKDs (≥55 years) with a median follow-up of 7.8 years showed no significant difference in major CV event risk compared to nondonors.
📍Mjøen et al. found a 40% higher risk of CV death among LKDs compared to healthy controls over a 15.1-year follow-up.
📍Of 224 deaths among 1,901 LKDs, 68 (30.4%) were due to CVD.
📍A follow-up study found a 64% higher risk of diagnosed IHD in LKDs compared to nondonors over a mean follow-up of > 10 years (adjusted OR, 1.64; 95% CI, 1.10 to 2.43).
📍↓ kidney function may be a risk factor for postdonation CV events and death, similar to patterns observed in the general population.
📍Currently, No evidence that predonation cardiac testing or risk factor modification beyond general guidelines reduces long-term CV risk for LKDs

(Financial Risks)
📍Counseling on financial risks is crucial for LKD candidates.
📍Even with medical expenses covered, LKDs may face costs for travel, meds, lost work time, and dependent care.
📍Long-term financial effects on employment and socioeconomic status are less understood.
📍🇰🇷 study found LKDs had↑employment loss (21.7% vs. 11.2%)
📍Candidates should receive region-specific counseling about financial costs and risks, and access to legitimate cost replacement programs is essential.
📍AST offers an online financial toolkit for donor candidates to understand financial aspects.
📍Their group published best practices to ↓ financial consequences for living donors.
livingdonortoolkit.com
📍Counseling on financial risks is crucial for LKD candidates.
📍Even with medical expenses covered, LKDs may face costs for travel, meds, lost work time, and dependent care.
📍Long-term financial effects on employment and socioeconomic status are less understood.
📍🇰🇷 study found LKDs had↑employment loss (21.7% vs. 11.2%)
📍Candidates should receive region-specific counseling about financial costs and risks, and access to legitimate cost replacement programs is essential.
📍AST offers an online financial toolkit for donor candidates to understand financial aspects.
📍Their group published best practices to ↓ financial consequences for living donors.
livingdonortoolkit.com
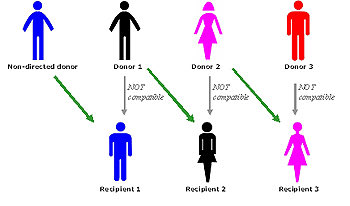
Kidney Paired Donation (KPD)
📍KPD programs help patients willing but biologically incompatible donors by exchanging kidneys among donor-recipient pairs.
📍KPD uses algorithms to match blood groups, donor-specific alloreactivity, and other characteristics for successful exchanges.
📍Non directed donors (donors without an identified recipient) expand the donor pool through chains of kidney exchanges.
📍KPD is the fastest growing LDKT modality, making up about 16% of LDKTs per year.
📍KPD is underused due to complexity, cost, patient and physician acceptance, and degree of allosensitization.
📍If all centers used KPD at the rate of high-performing centers, an additional 1000 transplants could be performed annually.
📍"Advanced donation" programs allow donors to give to the KPD pool in exchange for a "voucher" for future transplantation of their intended recipient.
📍The Mayo Clinic's 10-year KPD program experience showed reduced viral mismatches and improved transplant quality scores.
📍NKF calls for research to remove KPD barriers and improve matching algorithms.
📍Enhancing KPD participation is crucial for increasing access to LDKT.
📍KPD programs help patients willing but biologically incompatible donors by exchanging kidneys among donor-recipient pairs.
📍KPD uses algorithms to match blood groups, donor-specific alloreactivity, and other characteristics for successful exchanges.
📍Non directed donors (donors without an identified recipient) expand the donor pool through chains of kidney exchanges.
📍KPD is the fastest growing LDKT modality, making up about 16% of LDKTs per year.
📍KPD is underused due to complexity, cost, patient and physician acceptance, and degree of allosensitization.
📍If all centers used KPD at the rate of high-performing centers, an additional 1000 transplants could be performed annually.
📍"Advanced donation" programs allow donors to give to the KPD pool in exchange for a "voucher" for future transplantation of their intended recipient.
📍The Mayo Clinic's 10-year KPD program experience showed reduced viral mismatches and improved transplant quality scores.
📍NKF calls for research to remove KPD barriers and improve matching algorithms.
📍Enhancing KPD participation is crucial for increasing access to LDKT.
Loading suggestions...