1/☢️
Early on April 26, 1986, reactor 4 at the Chernobyl nuclear power plant in Northern Ukraine exploded, sending a radioactive plume into the atmosphere.
Public health authorities rushed to administer iodine in surrounding communities.
Why?
#medtwitter #tweetorial
Early on April 26, 1986, reactor 4 at the Chernobyl nuclear power plant in Northern Ukraine exploded, sending a radioactive plume into the atmosphere.
Public health authorities rushed to administer iodine in surrounding communities.
Why?
#medtwitter #tweetorial
2/
After the explosion, a man fishing nearby saw a blue glow emanating from the site, which represented ionizing radiation fluorescing molecules in the air.
Released radionuclides included iodine-131, caesium-134 and caesium-137.
tandfonline.com
After the explosion, a man fishing nearby saw a blue glow emanating from the site, which represented ionizing radiation fluorescing molecules in the air.
Released radionuclides included iodine-131, caesium-134 and caesium-137.
tandfonline.com
3/
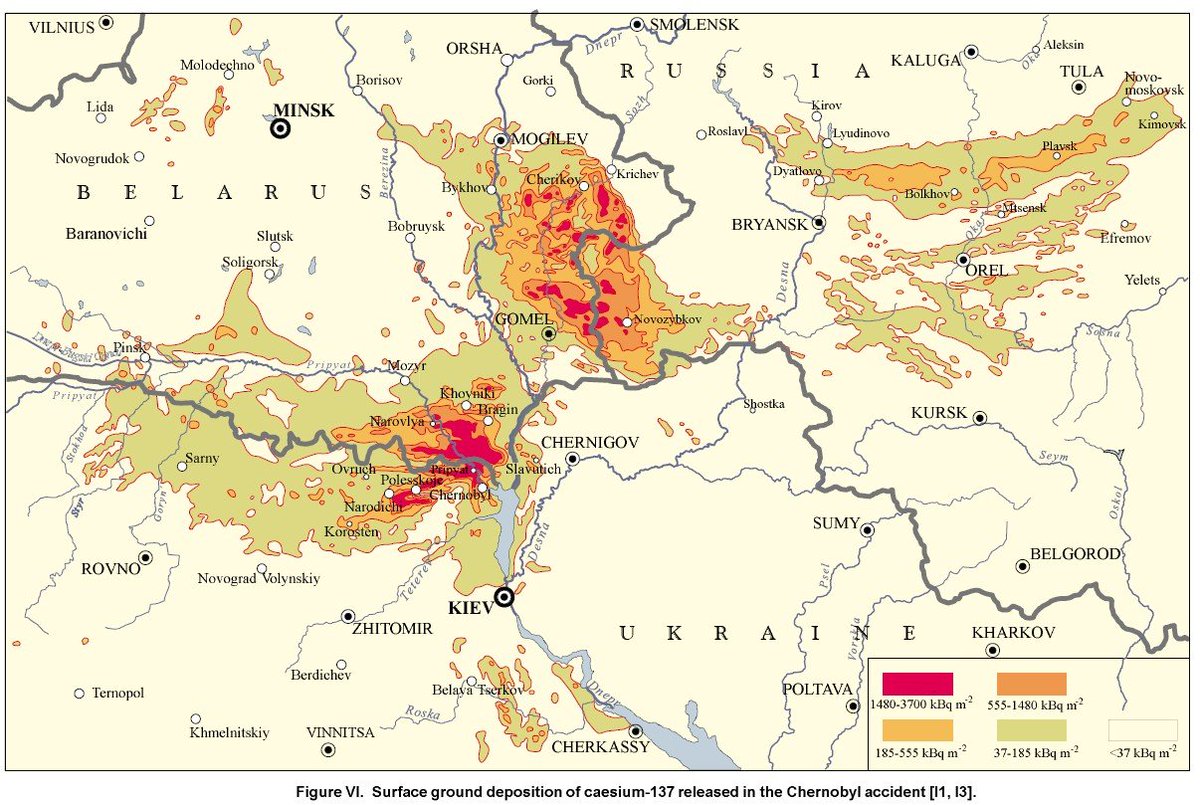
These radionuclides swept across a wide geographic area, radiating large swaths of Eastern Europe.
People were immediately exposed to radiation from breathing the air and, over the course of months, from consuming contaminated milk and vegetables.
unscear.org
These radionuclides swept across a wide geographic area, radiating large swaths of Eastern Europe.
People were immediately exposed to radiation from breathing the air and, over the course of months, from consuming contaminated milk and vegetables.
unscear.org
4/
This radiation exposure had numerous health impacts.
28 Chernobyl site workers died of radiation poisoning w/in 3 months. Thousands of recovery personnel had high dose radiation exposure w/⬆️ incidence of leukemia and cataracts in subsequent decades.
unscear.org
This radiation exposure had numerous health impacts.
28 Chernobyl site workers died of radiation poisoning w/in 3 months. Thousands of recovery personnel had high dose radiation exposure w/⬆️ incidence of leukemia and cataracts in subsequent decades.
unscear.org
5/
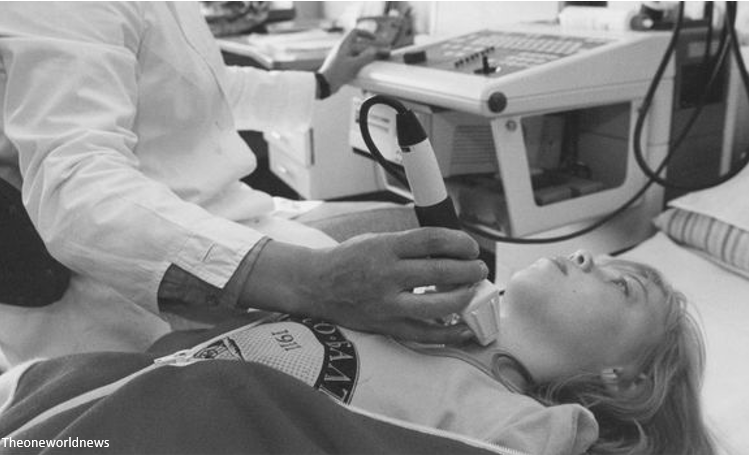
One of the most common long term effects of Chernobyl was thyroid cancer, particularly w/ children who drank radioactive milk in the months after the blast.
As of 2005, 6,000+ thyroid cancer cases had been linked to post-Chernobyl radiation exposure.
unscear.org
One of the most common long term effects of Chernobyl was thyroid cancer, particularly w/ children who drank radioactive milk in the months after the blast.
As of 2005, 6,000+ thyroid cancer cases had been linked to post-Chernobyl radiation exposure.
unscear.org
6/
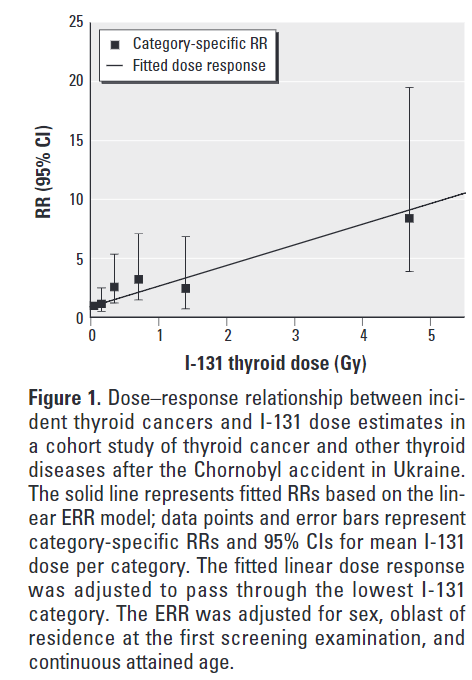
Subsequent research has demonstrated a linear dose-response relationship between the amount of iodine-131 exposure from Chernobyl and subsequent risk of developing thyroid cancer.
ehp.niehs.nih.gov
Subsequent research has demonstrated a linear dose-response relationship between the amount of iodine-131 exposure from Chernobyl and subsequent risk of developing thyroid cancer.
ehp.niehs.nih.gov
7/
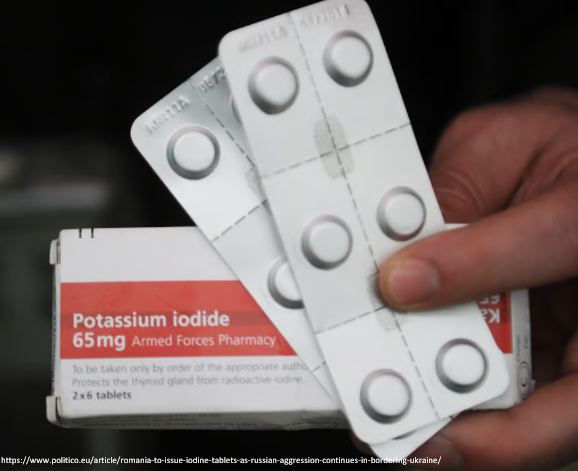
One of the first public health responses was to distribute potassium iodide pills/solutions to people in surrounding areas, w/ the goal of preventing thyroid cancers.
Poland, for example, distributed 18 million iodine doses to its at-risk population.
pubmed.ncbi.nlm.nih.gov
One of the first public health responses was to distribute potassium iodide pills/solutions to people in surrounding areas, w/ the goal of preventing thyroid cancers.
Poland, for example, distributed 18 million iodine doses to its at-risk population.
pubmed.ncbi.nlm.nih.gov
9/
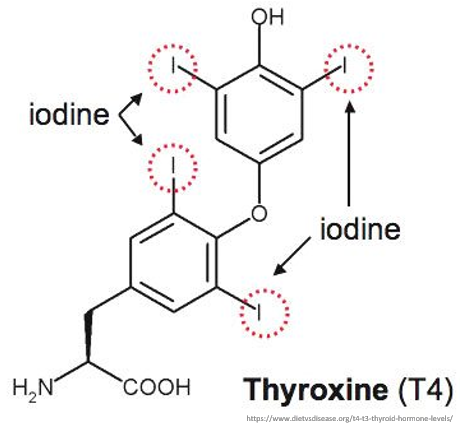
Iodine is an essential micronutrient, of which we need surprisingly little - only 5g of the element are enough to last a person for a lifetime.
Its main physiological purpose is as a core component of thyroid hormones T3 and T4.
pubmed.ncbi.nlm.nih.gov
Iodine is an essential micronutrient, of which we need surprisingly little - only 5g of the element are enough to last a person for a lifetime.
Its main physiological purpose is as a core component of thyroid hormones T3 and T4.
pubmed.ncbi.nlm.nih.gov
10/
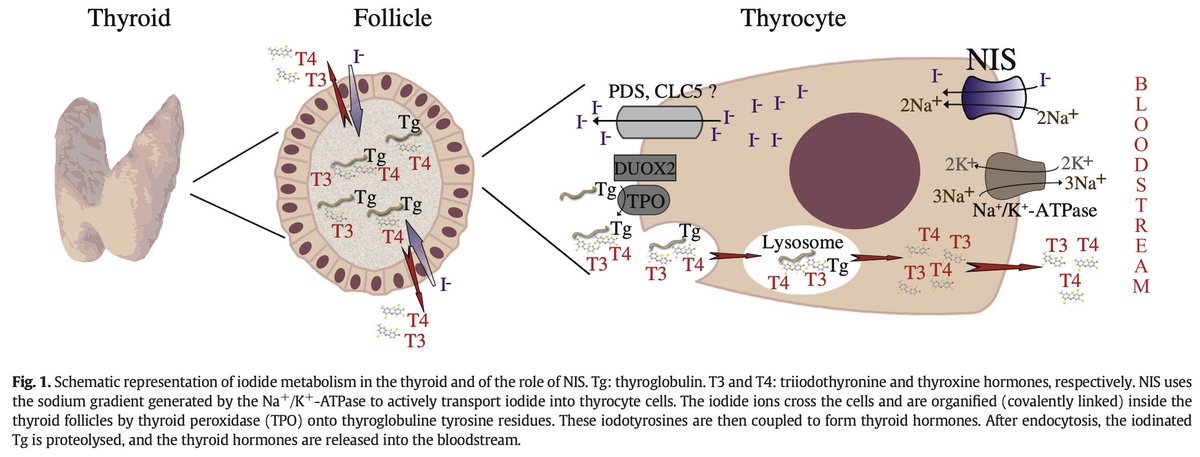
Iodine (in the form of the I- iodide anion) enters thyroid follicles via a sodium-iodide symporter known as NIS, or Natrium Iodide Symporter.
Once inside a follicle, I- gets incorporated into T3 and T4.
pubmed.ncbi.nlm.nih.gov
Iodine (in the form of the I- iodide anion) enters thyroid follicles via a sodium-iodide symporter known as NIS, or Natrium Iodide Symporter.
Once inside a follicle, I- gets incorporated into T3 and T4.
pubmed.ncbi.nlm.nih.gov
11/
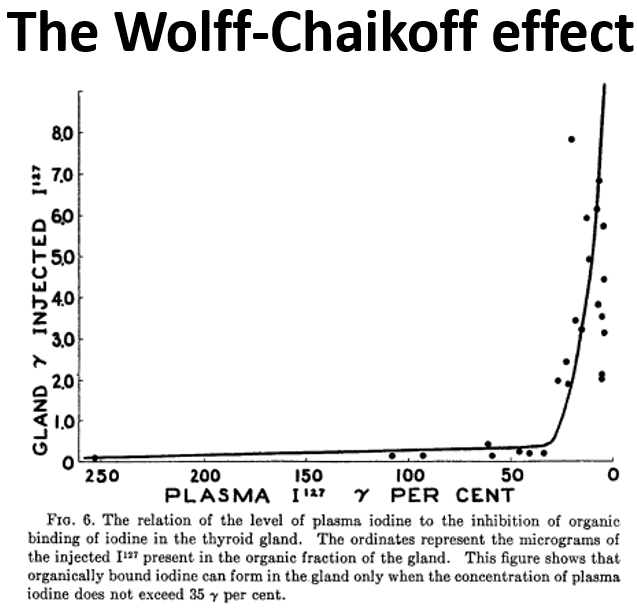
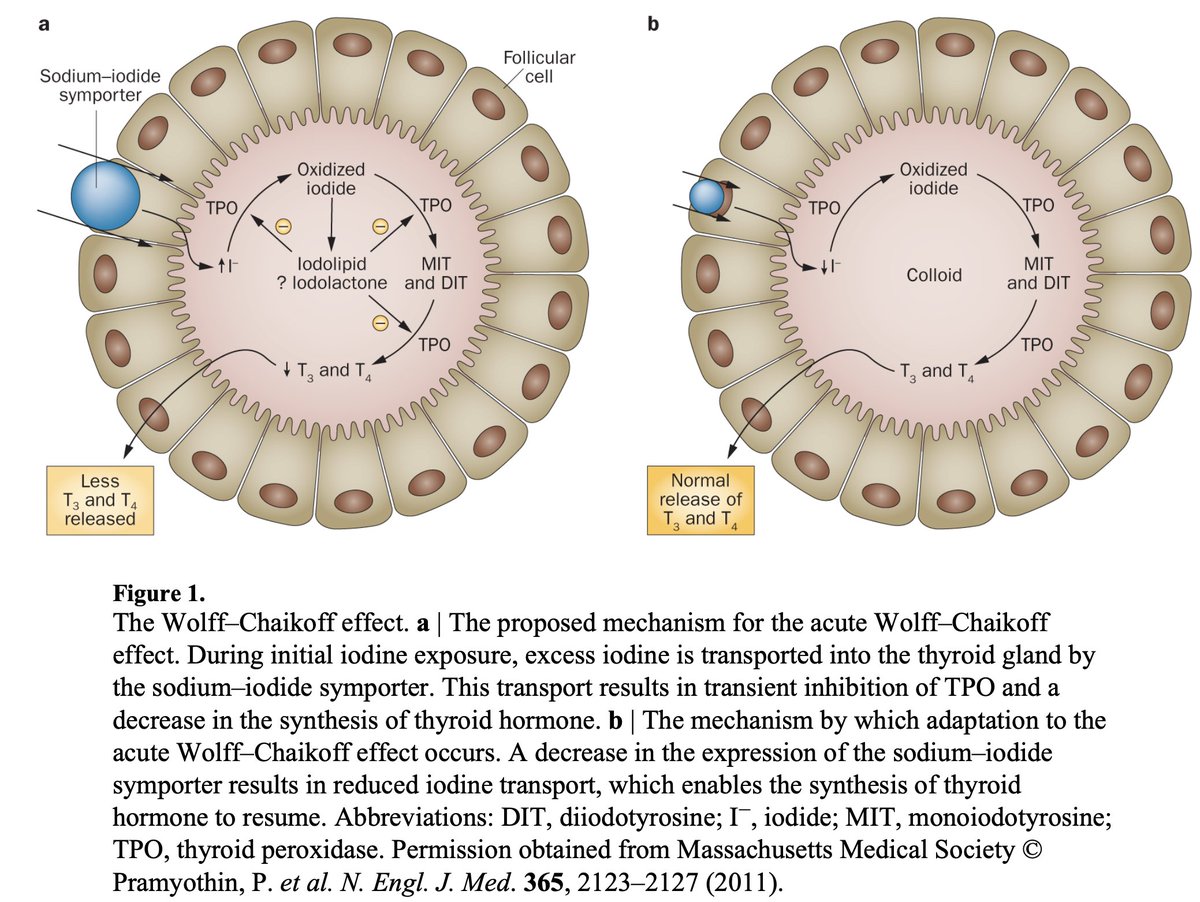
In 1948, Wolff and Chaikoff injected radioactive I-131 into rats, and found that elevated plasma iodine levels blocked the isotope from getting added into thyroid hormones.
This observation has since been called the Wolff-Chaikoff effect.
pubmed.ncbi.nlm.nih.gov
In 1948, Wolff and Chaikoff injected radioactive I-131 into rats, and found that elevated plasma iodine levels blocked the isotope from getting added into thyroid hormones.
This observation has since been called the Wolff-Chaikoff effect.
pubmed.ncbi.nlm.nih.gov
12/
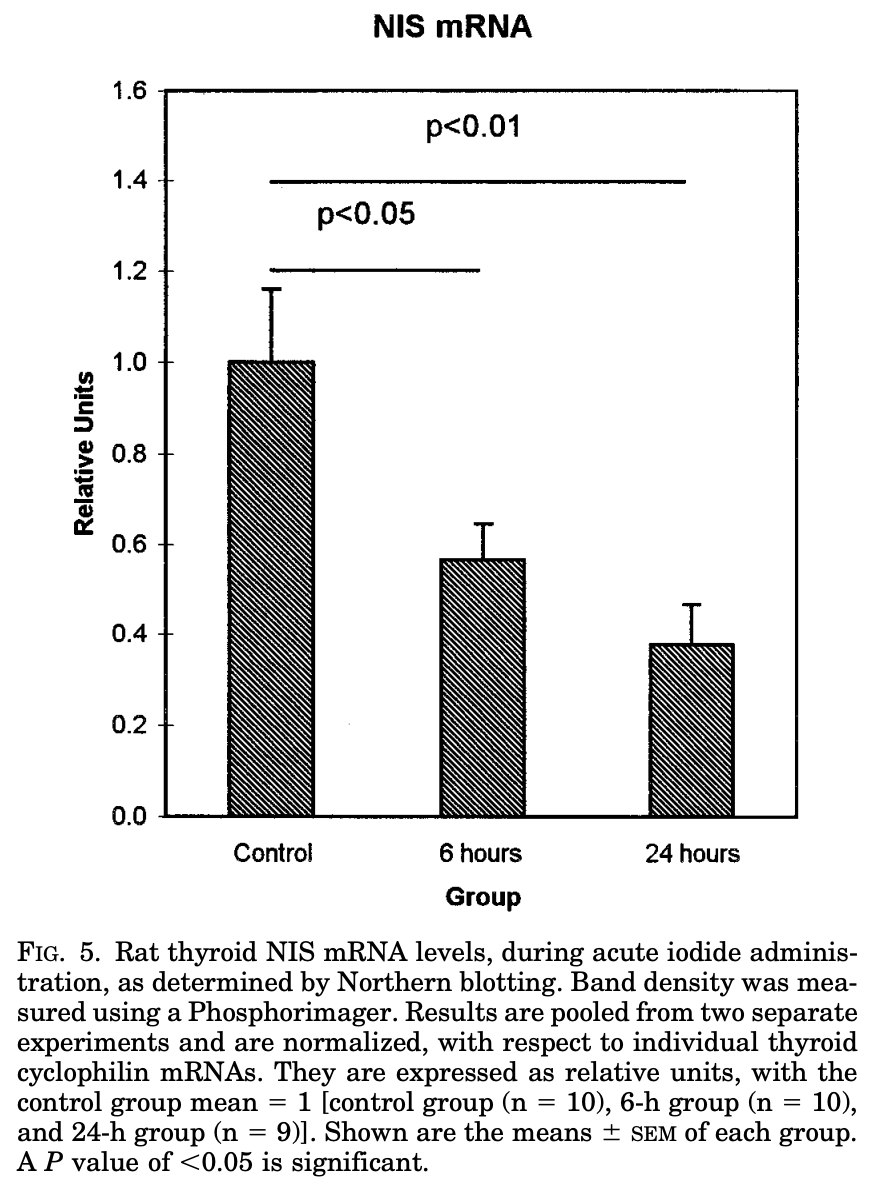
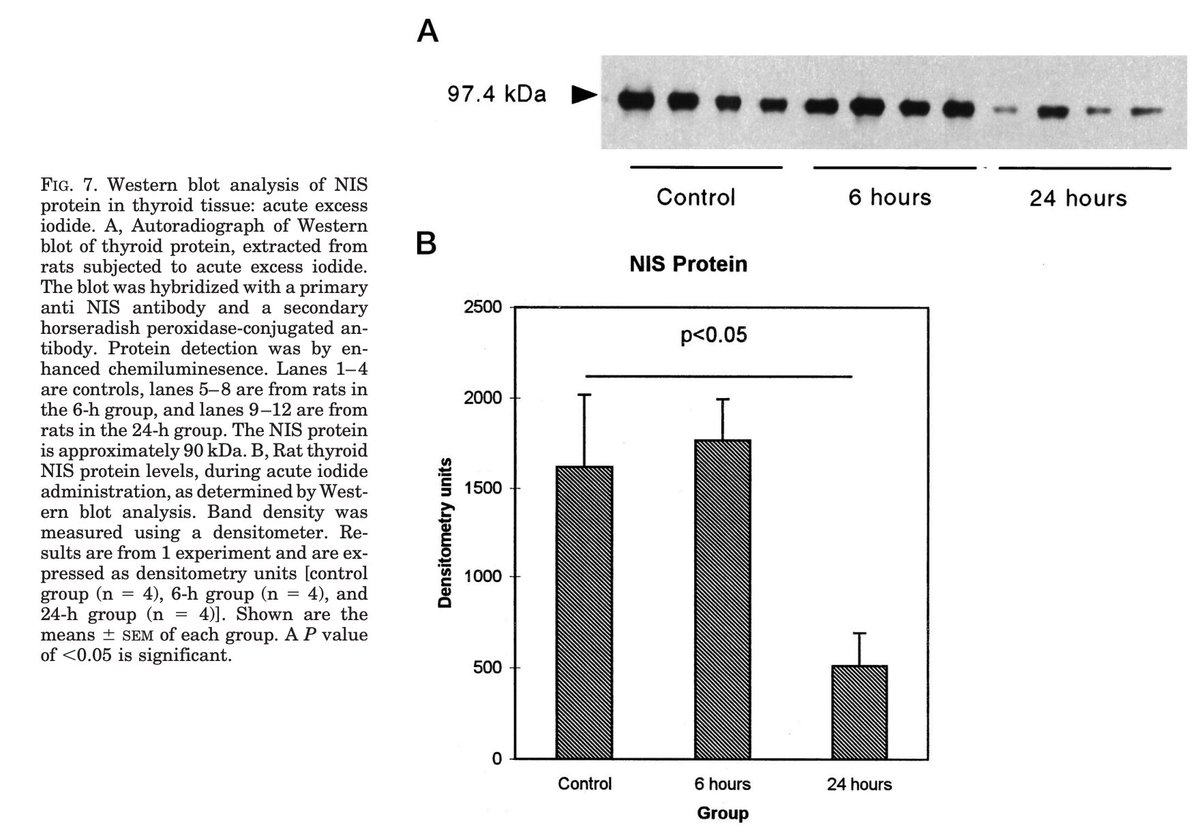
The mechanism of the Wolff-Chaikoff effect remains incompletely understood but animal experiments have suggested that it involves decreased NIS production (sodium-iodide symporter), blocking iodine uptake into the thyroid follicles.
pubmed.ncbi.nlm.nih.gov
The mechanism of the Wolff-Chaikoff effect remains incompletely understood but animal experiments have suggested that it involves decreased NIS production (sodium-iodide symporter), blocking iodine uptake into the thyroid follicles.
pubmed.ncbi.nlm.nih.gov
13/
In essence, the Wolff-Chaikoff effect is the thyroid's auto-regulatory response to exposure to high doses of iodine, maintaining homeostasis and avoiding excess thyroid hormone production until iodine levels in the thyroid fall back to normal.
pubmed.ncbi.nlm.nih.gov
In essence, the Wolff-Chaikoff effect is the thyroid's auto-regulatory response to exposure to high doses of iodine, maintaining homeostasis and avoiding excess thyroid hormone production until iodine levels in the thyroid fall back to normal.
pubmed.ncbi.nlm.nih.gov
14/
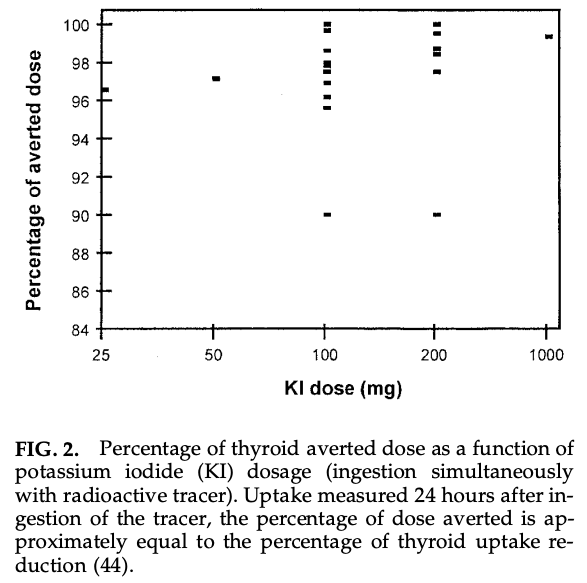
Because of the Wolff-Chaikoff effect, potassium iodide administration after Chernobyl would have blocked >95% of I-131 thyroid uptake in those who took it.
pubmed.ncbi.nlm.nih.gov
Because of the Wolff-Chaikoff effect, potassium iodide administration after Chernobyl would have blocked >95% of I-131 thyroid uptake in those who took it.
pubmed.ncbi.nlm.nih.gov
15/SUMMARY
☢️Radioactive I-131 was released from Chernobyl
☢️Potassium iodide pills/solution protected against thyroid cancer via the Wolff-Chaikoff effect
☢️Mechanism = ⬇️ sodium-iodide symporter production leading to ⬇️ thyroid iodine uptake
☢️Radioactive I-131 was released from Chernobyl
☢️Potassium iodide pills/solution protected against thyroid cancer via the Wolff-Chaikoff effect
☢️Mechanism = ⬇️ sodium-iodide symporter production leading to ⬇️ thyroid iodine uptake
جاري تحميل الاقتراحات...