Over the past 3 years I managed to publish multiple original research projects in top tiered journals using OPEN ACCESS DATA with ZERO funding.
Here is how I did it and how you can achieve the same impactful outcome, too.
#OpenAccess @NIH @nih_nhlbi
Here is how I did it and how you can achieve the same impactful outcome, too.
#OpenAccess @NIH @nih_nhlbi
Open access data in clinical research refers to data available for researchers to access and use. It includes a wide range of information gathered during clinical trials and epidemiology studies.
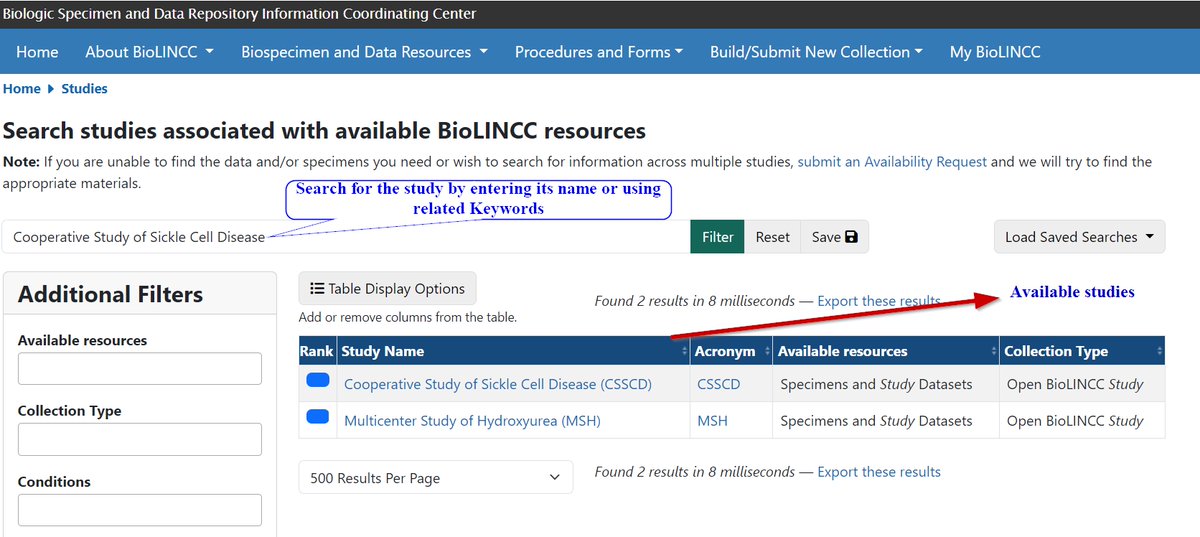
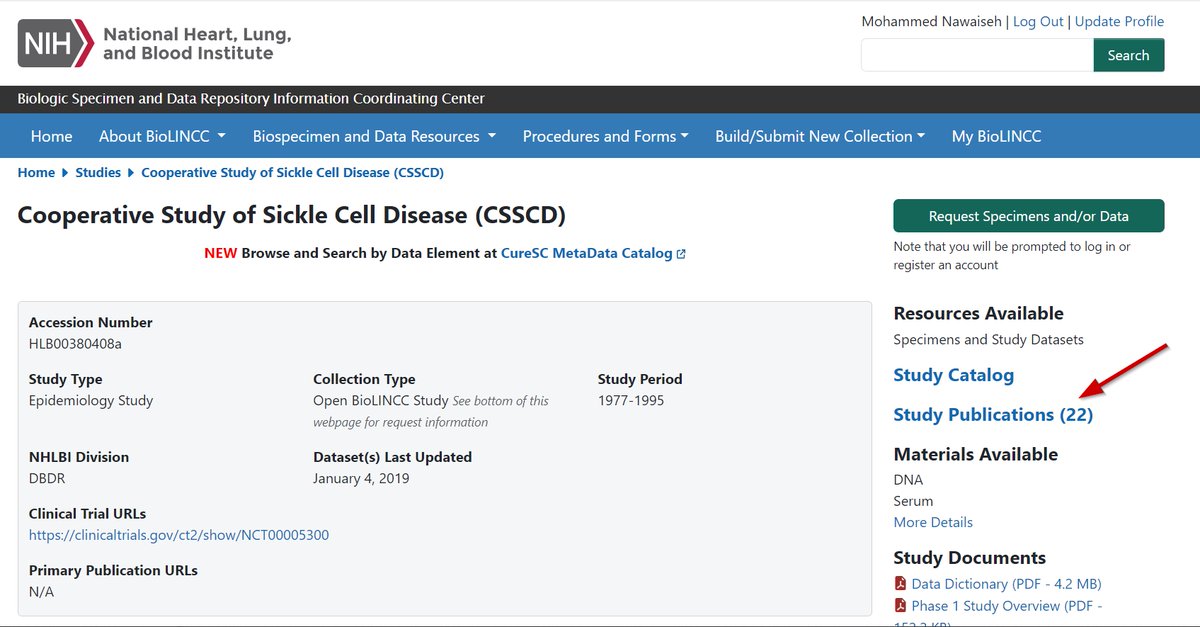
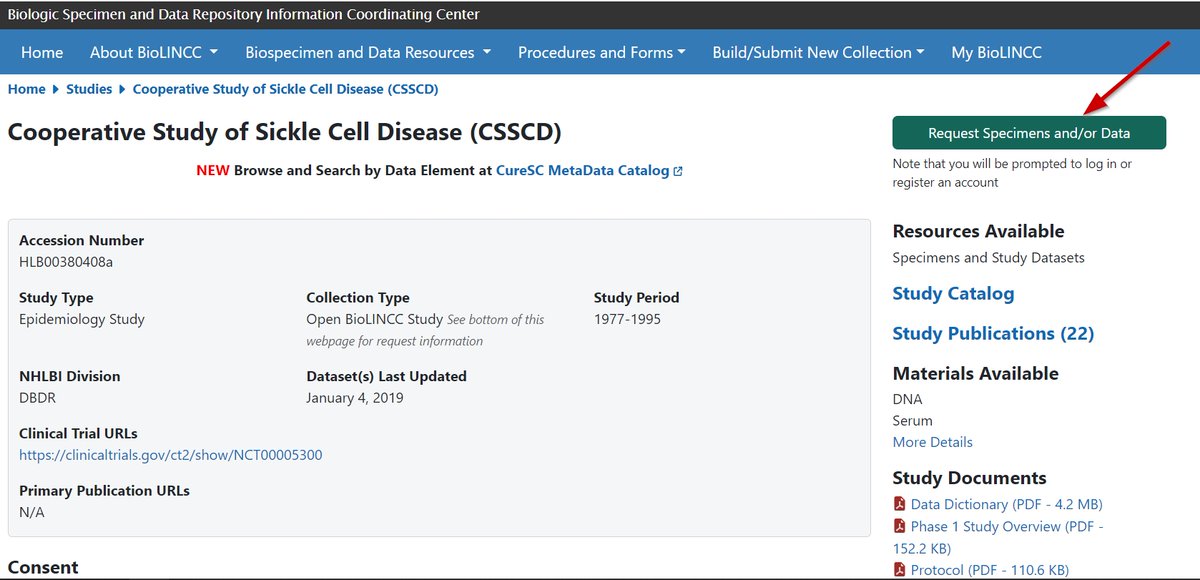
One of the largest and most comprehensive clinical data repositories is the National Institutes of Health (@NIH) funded Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC)
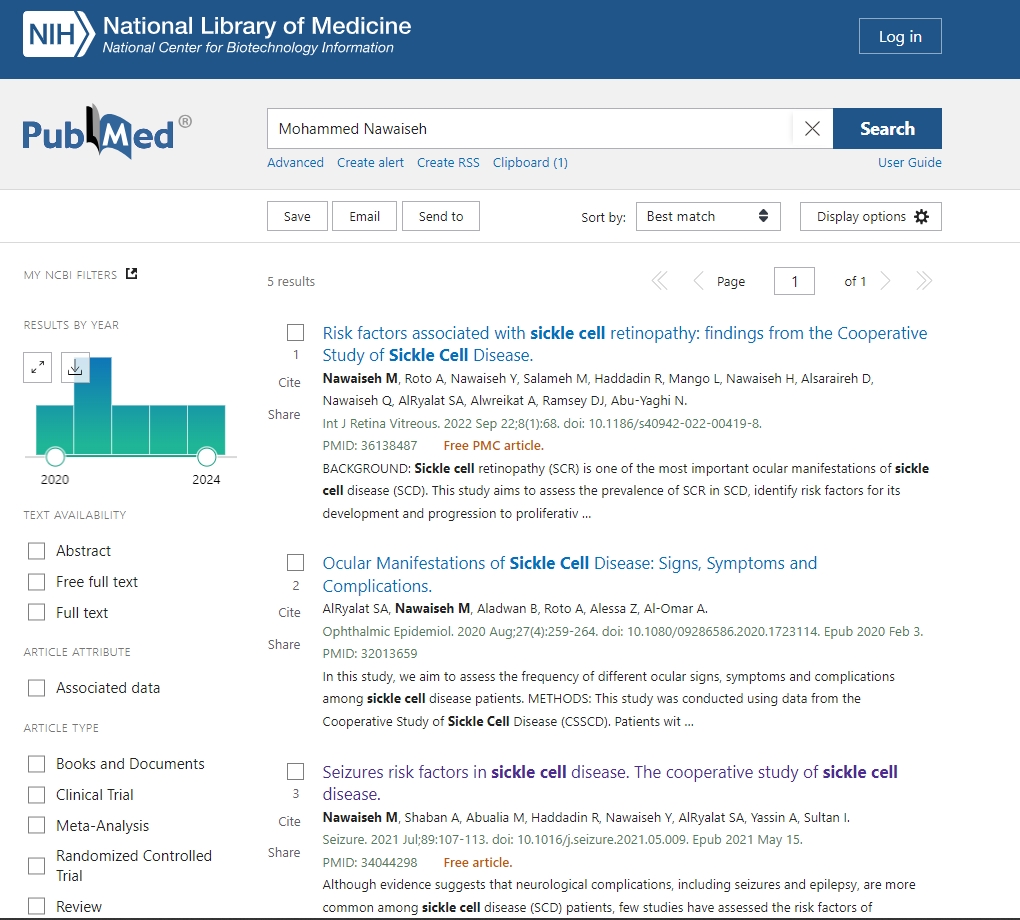
BioLINCC has more than 290 data sets available to be utilized and more than 1,100 articles have been published using data from its repository.
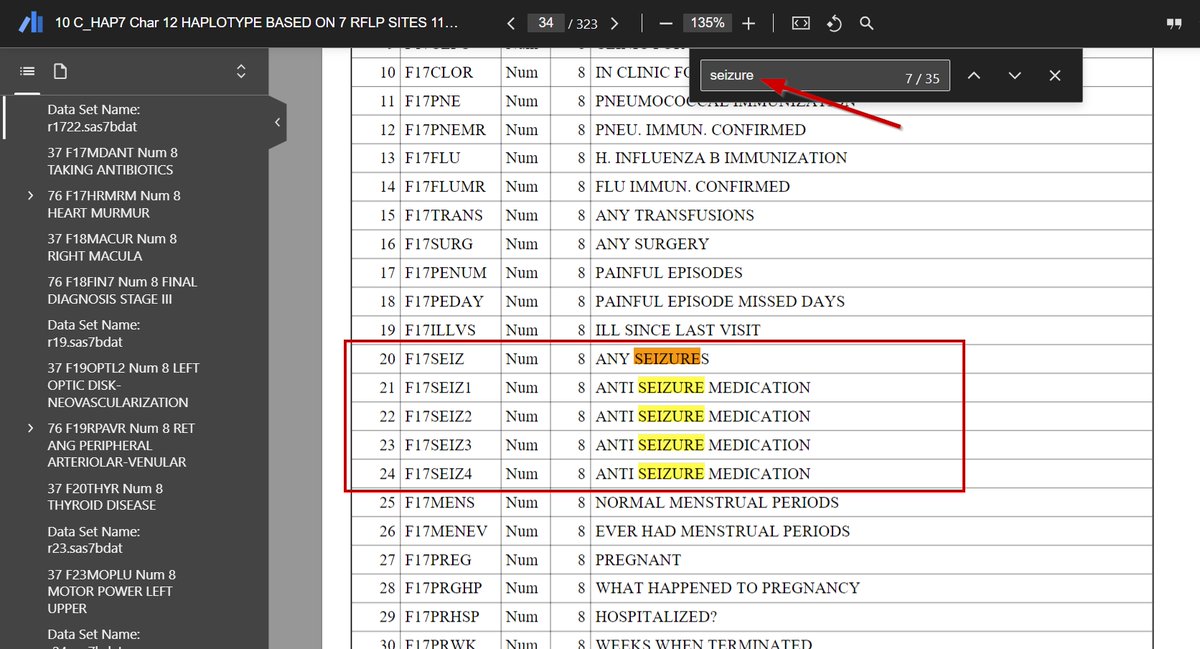
Here is a thread on how to Access studies data using the BioLINCC website.
Here is a thread on how to Access studies data using the BioLINCC website.
Here is the link for that study for more information on how we got the data and utilized it.
pubmed.ncbi.nlm.nih.gov
#neurotwitter
pubmed.ncbi.nlm.nih.gov
#neurotwitter
6.0 Files required
After filling the On-line request form, you will be asked to supply multiple files:
-Study Protocol (brief Literature review & methods)
-IRB Approval from the applicant’s institution (waiver, or expedited review, or full IRB).
-Author CV
-Study Abstract
After filling the On-line request form, you will be asked to supply multiple files:
-Study Protocol (brief Literature review & methods)
-IRB Approval from the applicant’s institution (waiver, or expedited review, or full IRB).
-Author CV
-Study Abstract
7.0 After initial acceptance, you will be asked to fill the Research Materials Distribution Agreement (RMDA). This agreement is generated by the website based on the provided information.
8.0 After final acceptance of your request, you will be notified when the secure data link is ready for access.
Finally, you will need to update the website of your progress yearly on March 1 and delete all the data after you finish your study.
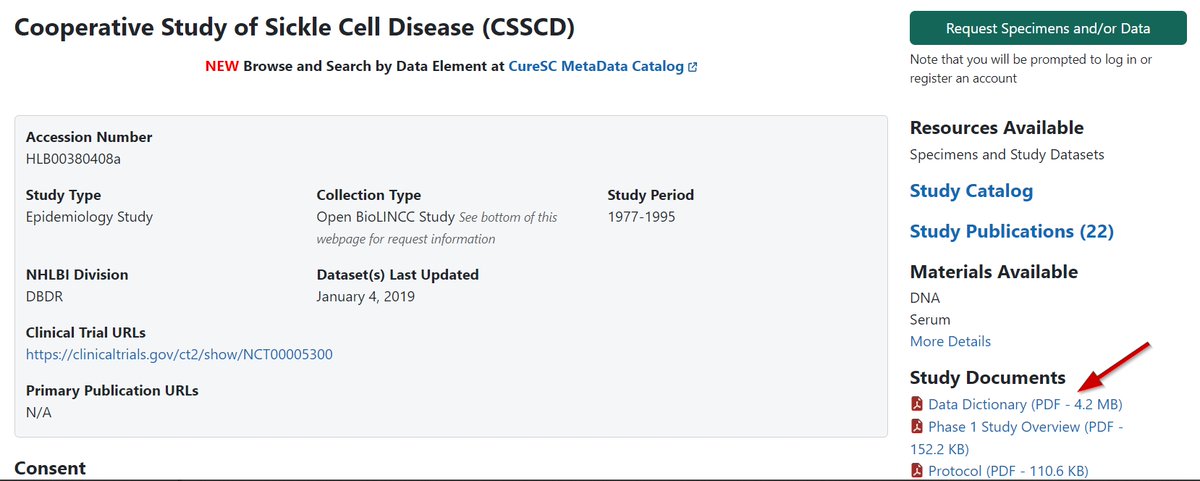
Feel free to reach out with any questions, or if you're interested, access the BioLINCC Handbook through this link: biolincc.nhlbi.nih.gov - a comprehensive guide to leveraging open access data from @nih_nhlbi studies.
Loading suggestions...