🧵 A FAILED PRODUCT FROM THE BEGINNING
Just a reminder that the stated purpose of the Pfizer Covid BNT162b2 (mRNA) vaccine was, according to the TGA's Product Assessment Report,
"Active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, in individuals 16 years of age and older."
When the product failed to do the one thing it was approved to do - which, in the Australian Immunisation Handbook, is called "vaccine failure" - the story changed.
'But, but, it was only ever meant to reduce the severity illness and death!'
Not true. Its stated purpose was prevention of COVID-19 disease.
Don't allow history to be rewritten to suit those who stand to profit from it. The product did not do the thing it was supposed to do. It's a failed product.
tga.gov.au
Just a reminder that the stated purpose of the Pfizer Covid BNT162b2 (mRNA) vaccine was, according to the TGA's Product Assessment Report,
"Active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, in individuals 16 years of age and older."
When the product failed to do the one thing it was approved to do - which, in the Australian Immunisation Handbook, is called "vaccine failure" - the story changed.
'But, but, it was only ever meant to reduce the severity illness and death!'
Not true. Its stated purpose was prevention of COVID-19 disease.
Don't allow history to be rewritten to suit those who stand to profit from it. The product did not do the thing it was supposed to do. It's a failed product.
tga.gov.au
2/ BTW this applies worldwide.
Table 1: International Regulatory Status shows that for a range of countries, the Pfizer Covid vaccine was approved for PREVENTION OF DISEASE.
tga.gov.au
Table 1: International Regulatory Status shows that for a range of countries, the Pfizer Covid vaccine was approved for PREVENTION OF DISEASE.
tga.gov.au
3/ As late as October 2021, PREVENTION OF DISEASE was still the purported purpose of the Pfizer Covid vaccine.
At the same time, booster doses were already being referred to in the Product Assessment report, even though State Government and Public Health officials were promising Australians that a 2 dose vaccine mandate would buy back their confiscated civil liberties.
tga.gov.au
At the same time, booster doses were already being referred to in the Product Assessment report, even though State Government and Public Health officials were promising Australians that a 2 dose vaccine mandate would buy back their confiscated civil liberties.
tga.gov.au
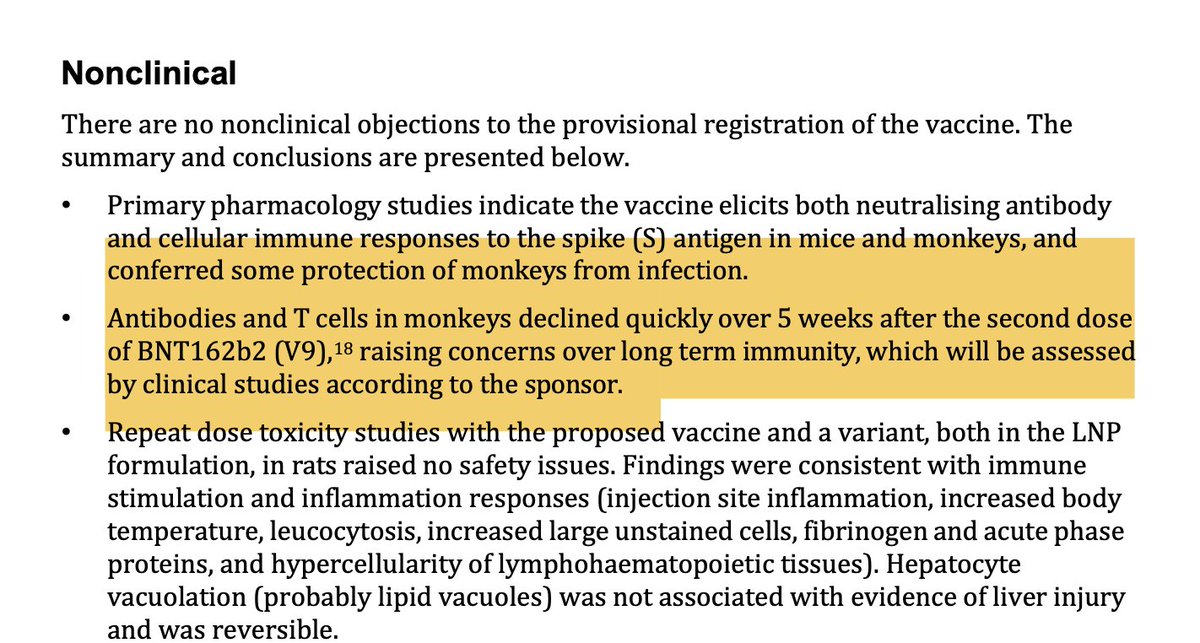
4/ Back in January 2021 it was already acknowledged in the TGA's Product Assessment Report that the 'vaccine' probably worked for 5 weeks at best.
This was not communicated to the public.
"Antibodies and T cells in monkeys declined quickly over 5 weeks after the second dose of BNT162b2 (V9),18 raising concerns over long term immunity, which will be assessed by clinical studies according to the sponsor."
tga.gov.au
This was not communicated to the public.
"Antibodies and T cells in monkeys declined quickly over 5 weeks after the second dose of BNT162b2 (V9),18 raising concerns over long term immunity, which will be assessed by clinical studies according to the sponsor."
tga.gov.au
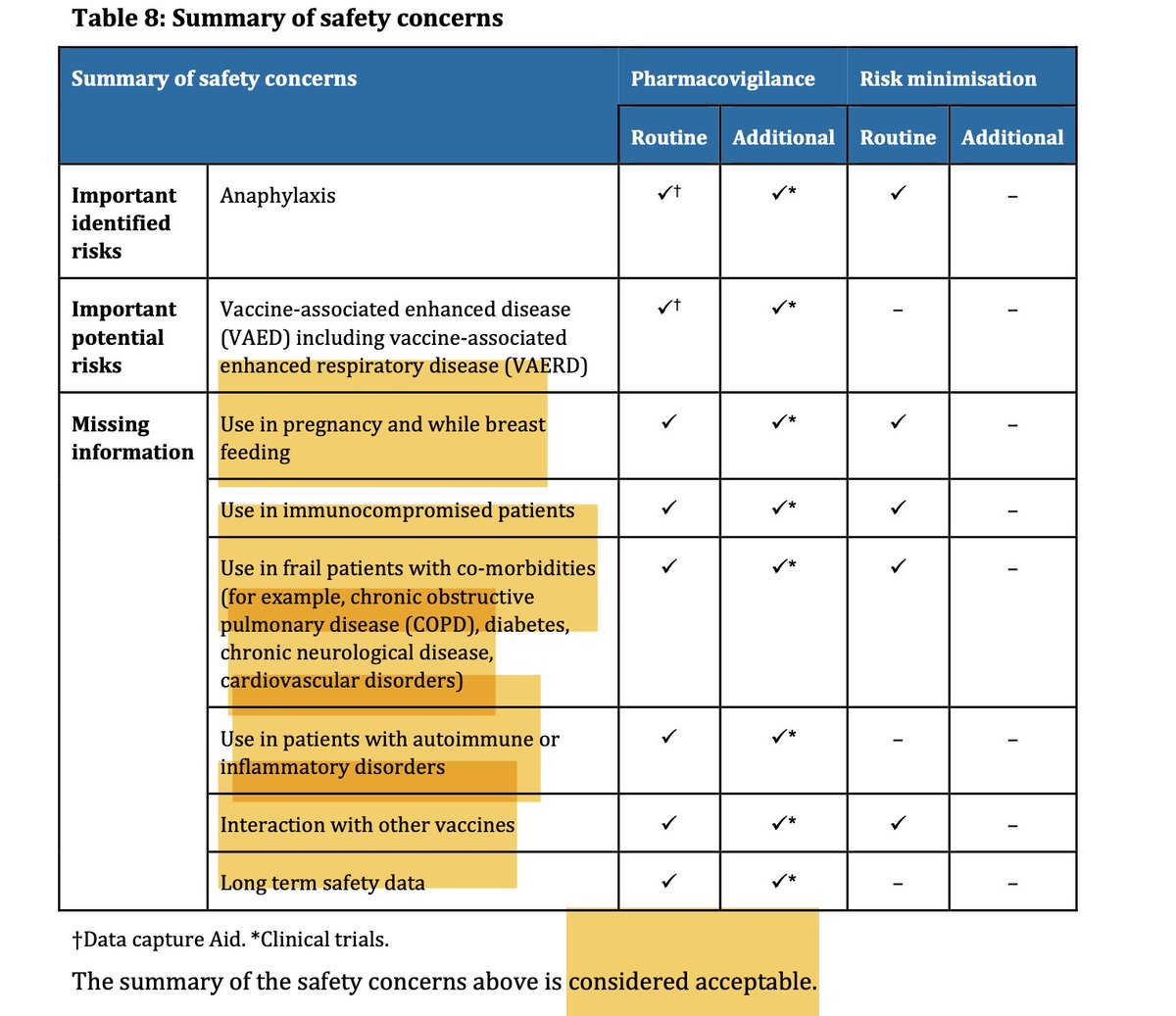
5/ Missing safety information at the time of provisional approval of the Pfizer Covid vaccine in Australia:
> Pregnancy & breastfeeding
> Immunocompromised patients
> Frail patients with co-morbidities
> Patients with autoimmune or inflammatory disorders
> Interaction with other vaccines
> Long term safety data
The TGA considered this "acceptable" and the vaccine rollout was prioritised to these groups (& healthcare workers) despite there being no safety data available.
Patients in these groups seeking exemptions due to the absence of safety data were denied.
tga.gov.au
> Pregnancy & breastfeeding
> Immunocompromised patients
> Frail patients with co-morbidities
> Patients with autoimmune or inflammatory disorders
> Interaction with other vaccines
> Long term safety data
The TGA considered this "acceptable" and the vaccine rollout was prioritised to these groups (& healthcare workers) despite there being no safety data available.
Patients in these groups seeking exemptions due to the absence of safety data were denied.
tga.gov.au
6/ And despite aggressive vaccine mandates purported to prevent transmission (Stop the Spread!) of Covid, the Product Assessment report clearly states that "vaccine efficacy against asymptomatic infection and viral transmission" had "not yet been addressed."
tga.gov.au
And yes, they said it would stop transmission.
news.com.au
tga.gov.au
And yes, they said it would stop transmission.
news.com.au
7/ Of course, all of this is irrelevant to a degree because the Pfizer vaccine tested in the famous RCT was a different product to the one that was rolled out to the public.
The rollout product was never tested at scale in an RCT before being approved.
The rollout product was never tested at scale in an RCT before being approved.
With thanks to @SenatorRennick for bringing this to the surface in a recent Senate Committee hearing.
aph.gov.au
aph.gov.au
Loading suggestions...