56 year old 👨🏼
PMH: obesity and GERD
😣 Develops abdominal discomfort
🍛 early satiety
⬇️ 20lb weight loss
🩻 CT: Gastric thickening and bilobar liver lesions
🤨 What would you do?
🧪CEA increased to 200 ng/mL
🔦EGD: ulcerated mass in body of stomach
🔬Path: poorly differentiated adenocarcinoma
🧪Initial IHC: pMMR and HER2 negative and PD-L1 CPS 1
🤔 What additional tests would you do next?
#GI21 #GastricCancer
While waiting for results...
👨🏼Discuss clinical trial options and screen for anti-CLDN18.2 based studies
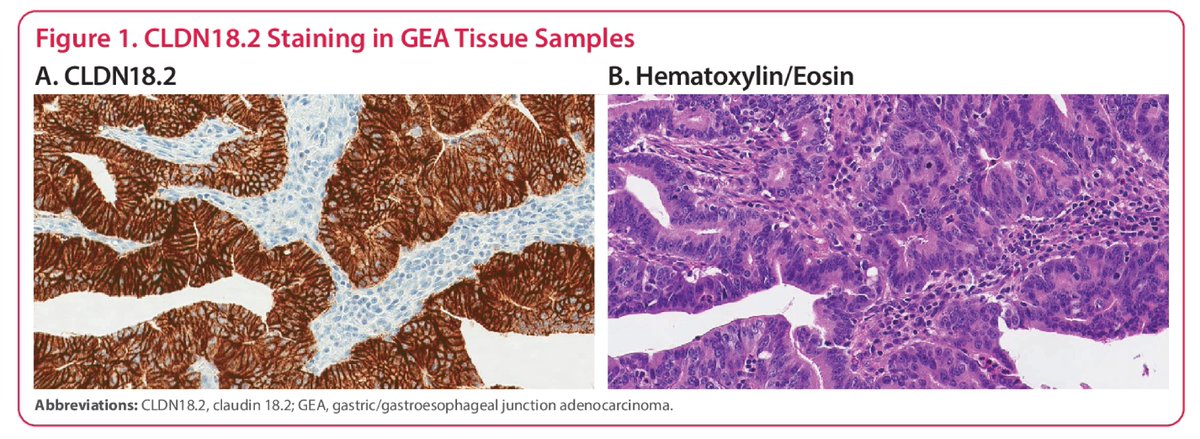
IHC following central review: positive >75% staining
📚@KlempnerSam #ASCOGI21
👨🏼🏫Mini tweetorial 1 👨🏻🏫
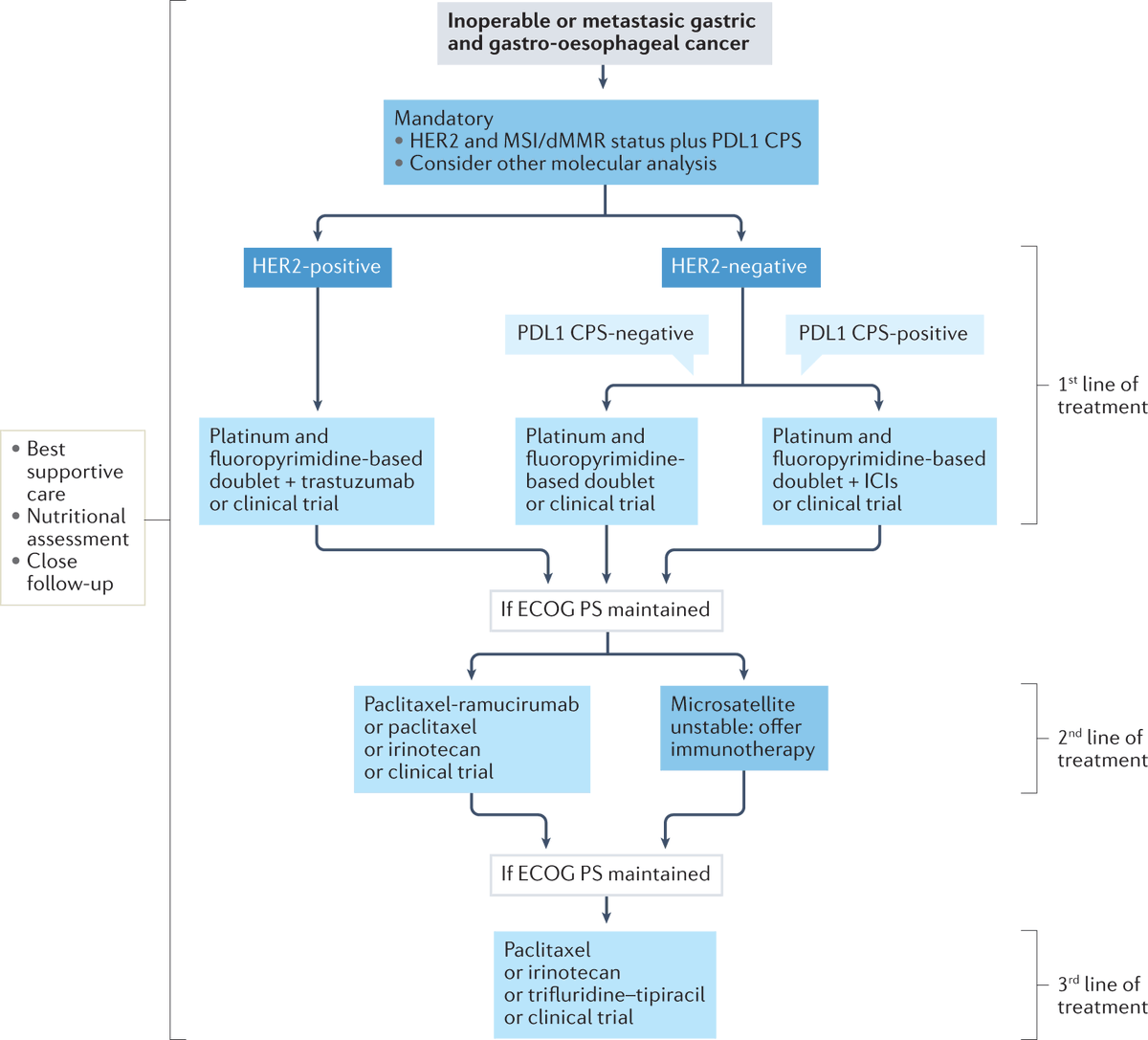
📌SOC systemic tx for metastatic #GastricCancer
‼️Always consider clinical trials‼️
🧬Biomarkers drive therapy
✨NEW✨ 8/2023 @NCCN to include 1L IO for dMMR/MSI-H (Finally🙌🏽)
📚#MariaAlsina @TaberneroJosep nature.com
👨🏼🏫Mini tweetorial 2👨🏻🏫
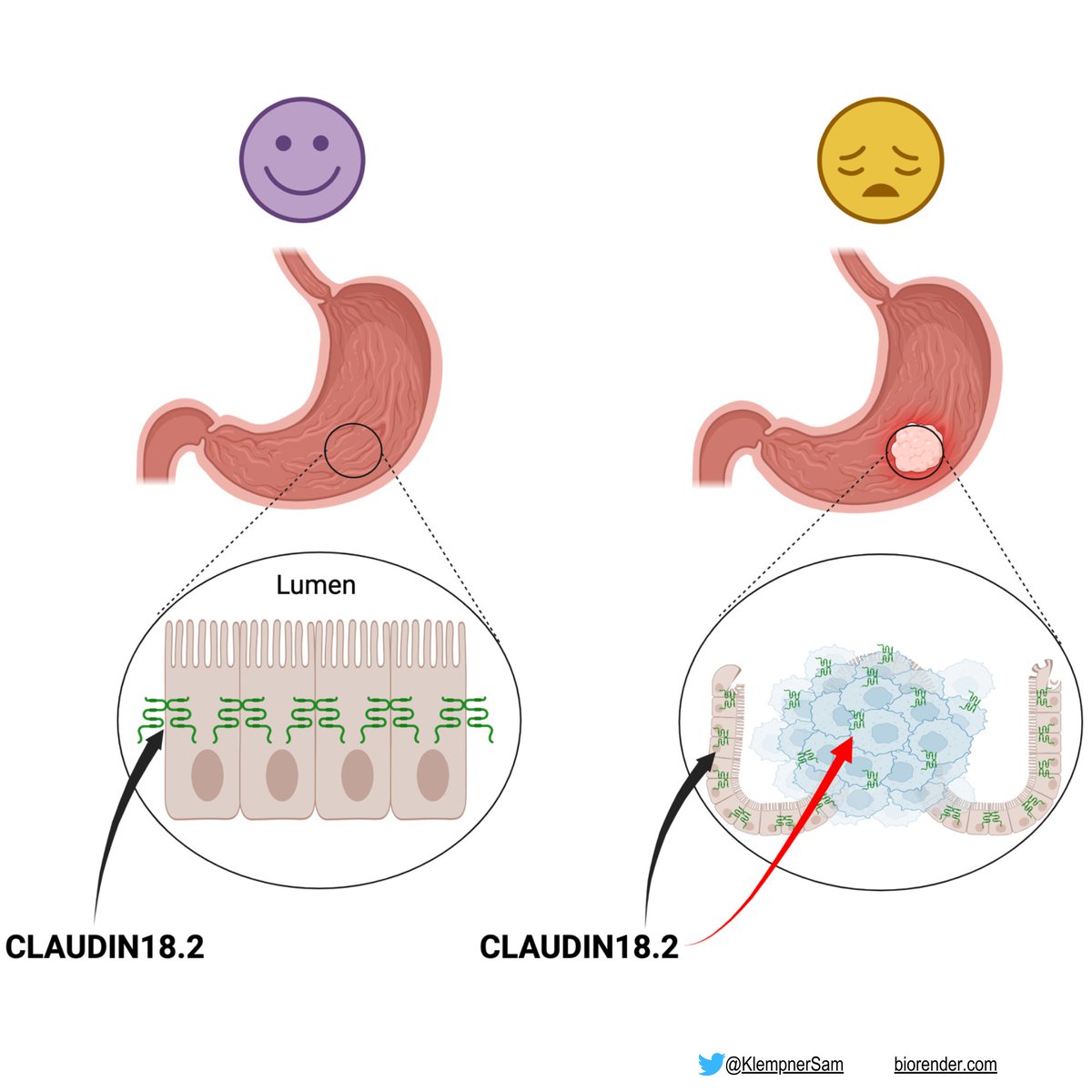
📌CLAUDIN 18.2
📍Transmembrane tight junction in gastric epithelia
❌Not routinely in norm tissue outside gastric mucosa (cancer-restricted antigen)
📍expressed in tumor types
🔸Gastric
🔹GEJ
🔸Biliary
🔹Pancreas
👨🏼🏫Mini tweetorial 3
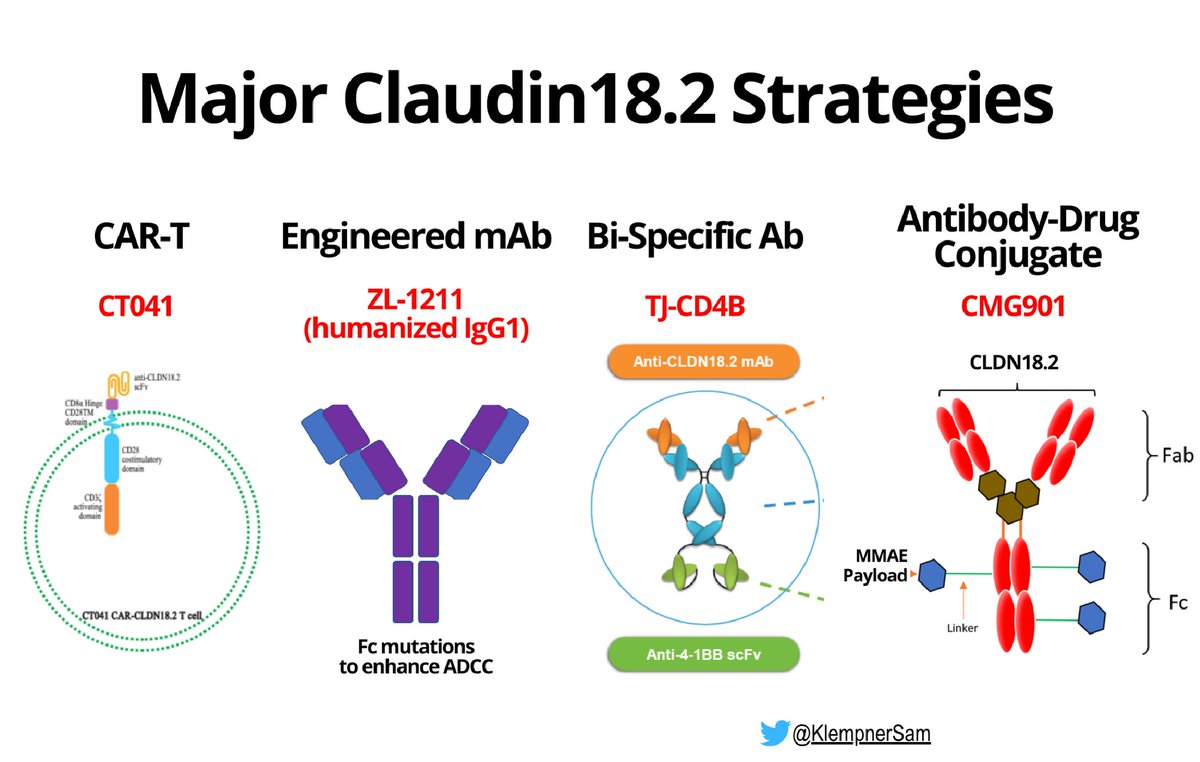
✨Claudin18.2 (CLDN18.2)
🎯Tumor intrinsic surface targets - multi therapeutic approaches
🎯Avail strategies
Engineered mAb
Bispecific Ab
Antibody-Drug
CAR-T
🪚If you can hit it, you can kill it
📚Cao Biomark Res
biomarkerres.biomedcentral.com
👨🏼🏫Mini tweetorial 4👨🏻🏫
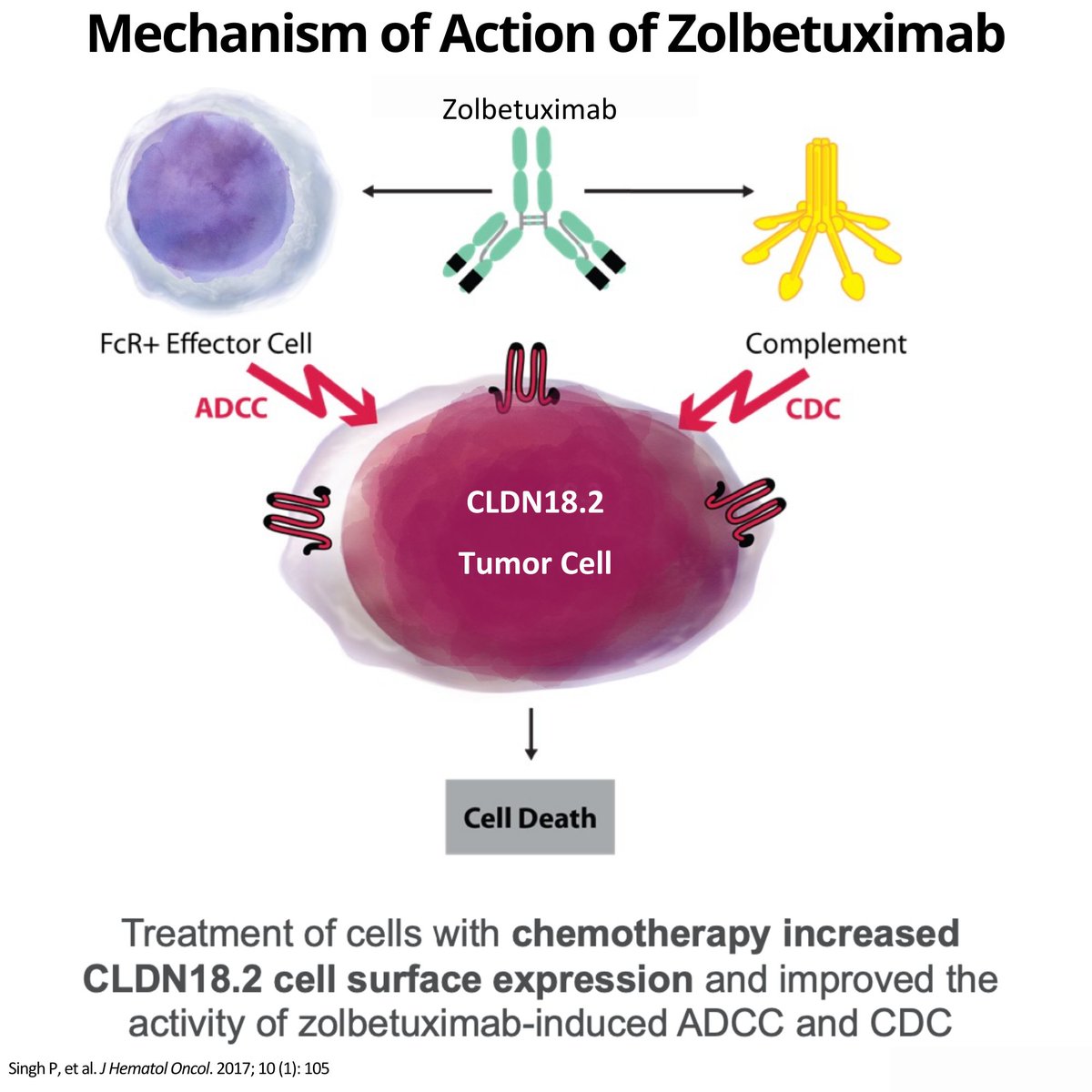
📌Zolbetuximab
👨🏼enrolled ph3 Zolbe+ox chemo
⚛️MOA
🎯1st-in-class chimeric IgG1 mAb target CLDN18.2 inducing antibody & complement dependent cytotox
🔹studied
mono
combo + 1L chemo
combo + anti-PD-1
📚@KoheiShitara #ASCOGI23 #GI23
👨🏼🏫Mini tweetorial 5
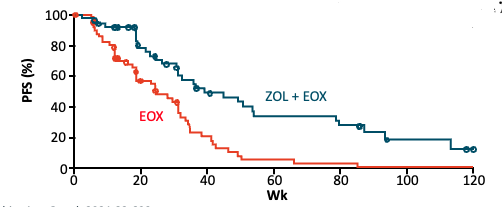
✨FAST
📍Ph2
🔹Zolbe +/- EOX
👍🏽PFS: intermed/High express (>40%) mPFS 7.5 v 5.3 mos HR (95% CI) 0.44 (0.29-0.67); P<.0005
Greater benefit➡️IHC express cut off >70% 9 v 5.7 mos HR 0.38 (0.23-0.62) P<0.005
📚#Sahin annalsofoncology.org
👨🏼🏫Mini tweetorial 6👨🏻🏫
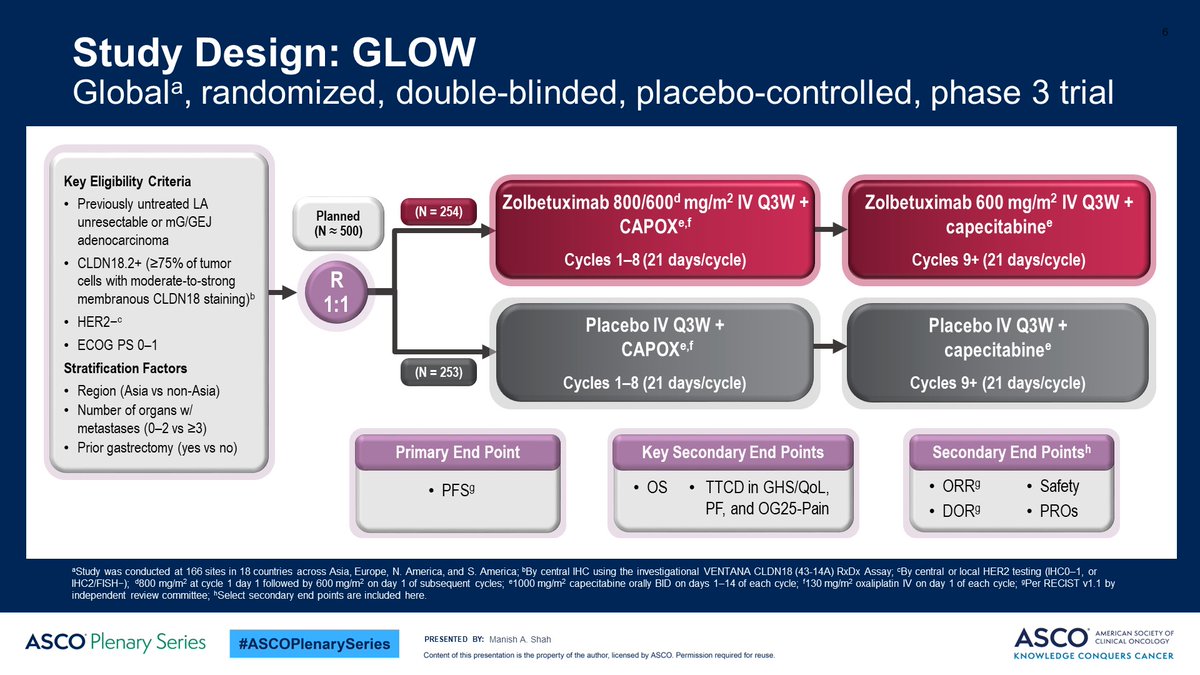
🕯️GLOW
🎲Ph3: double blind placebo-control
CLDN18.2+ (≥75% by IHC) HER2-neg unresectable/met G/GEJ
adenoca
no prior CT
📍N=507
CAPOX + Zolbe/Placebo
1° endpt: IRC-assessed PFS
2° endpt: OS, ORR, DoR, safety, PK, QoL
📚@mdmanishshah
👨🏼🏫Mini tweetorial 7👨🏻🏫
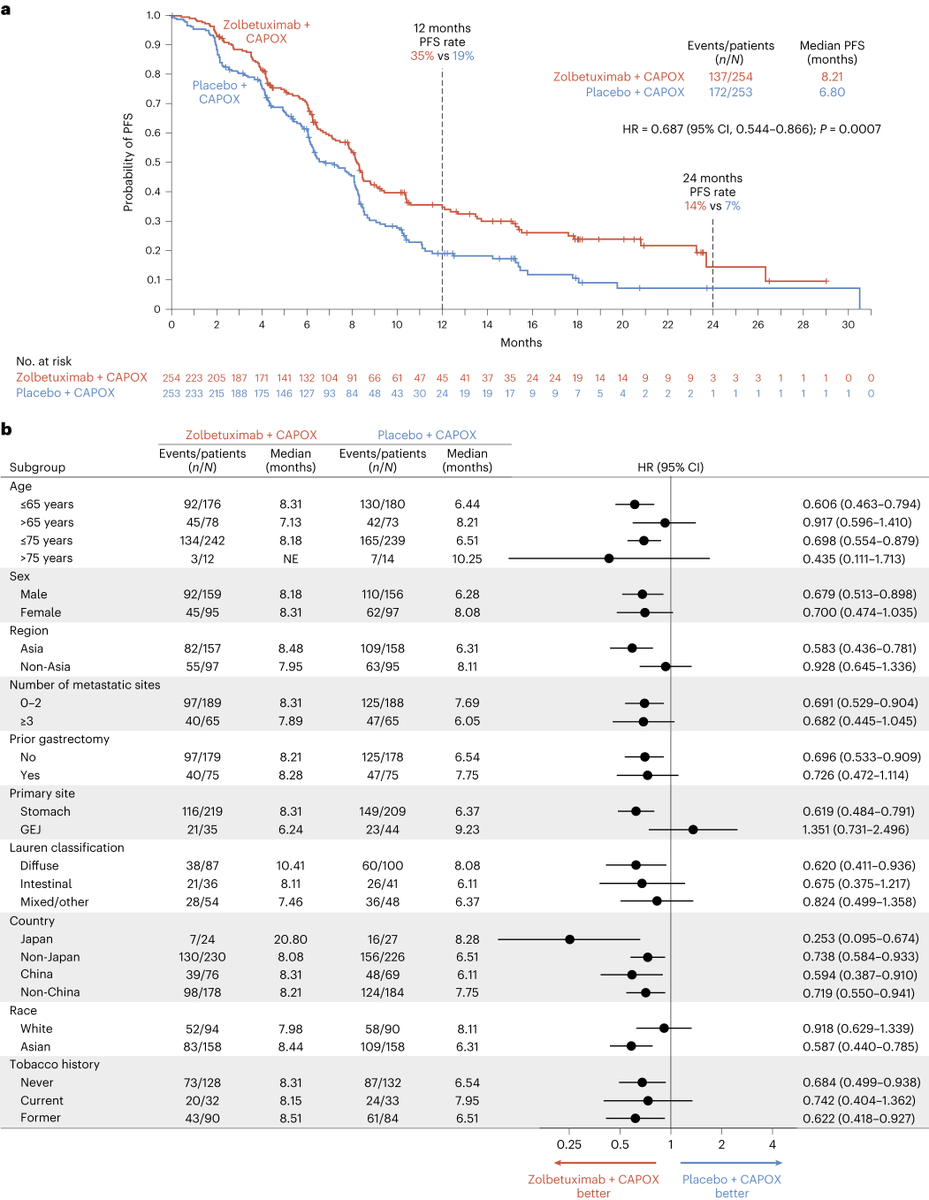
✨GLOW
1° endpt: mPFS 8.21 mos v 6.80 mos with zolbe v placebo
HR = 0.687
95% (CI) 0.544–0.866
P = 0.0007
🗝️2° endpt: OS (median, 14.39 mos v 12.16 mos; HR = 0.771; 95% CI, 0.615–0.965; P = 0.0118)
📚nature.com
👨🏼🏫Mini tweetorial 8👨🏻🏫
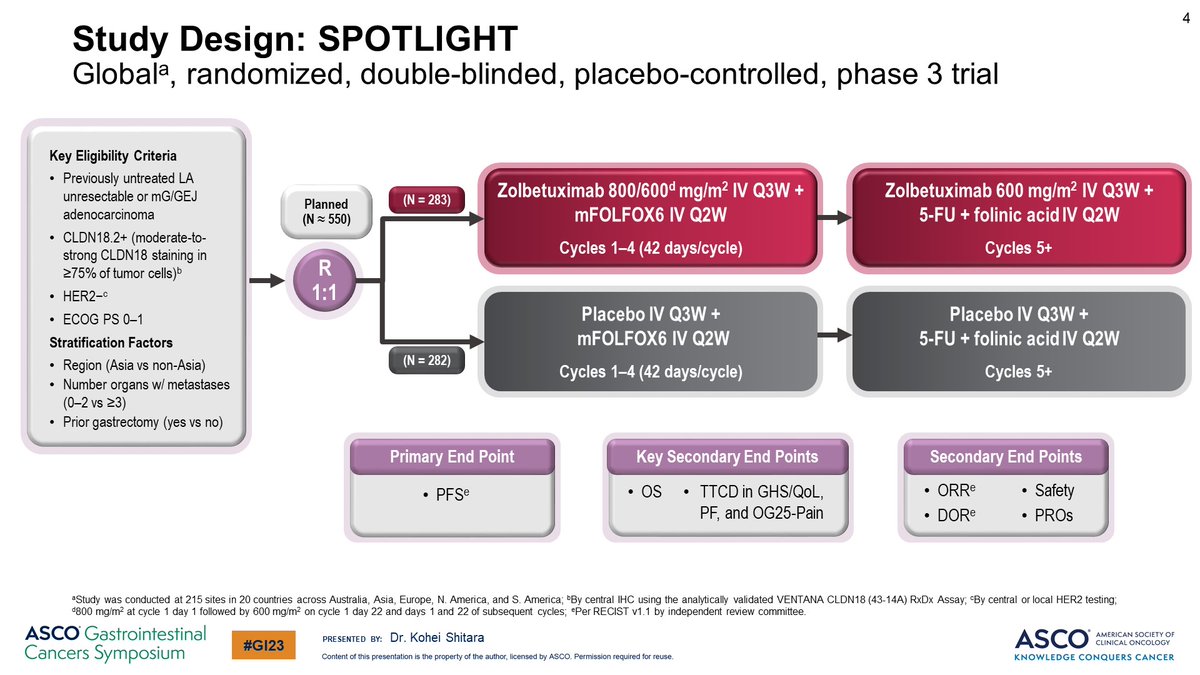
✨SPOTLIGHT
🎲Double blind placebo control
📍ph3
FOLFOX +Zolbe/placebo
1° endpt: PFS
2° endpt: OS, TTCD (GHS/QoL, PF, & QLQ-OG25-Pain score)
++ endpts: ORR, DoR, safety, PROs
📚@KoheiShitara
doi.org
#ASCOGI23
👨🏼🏫Mini tweetorial 9👨🏻🏫
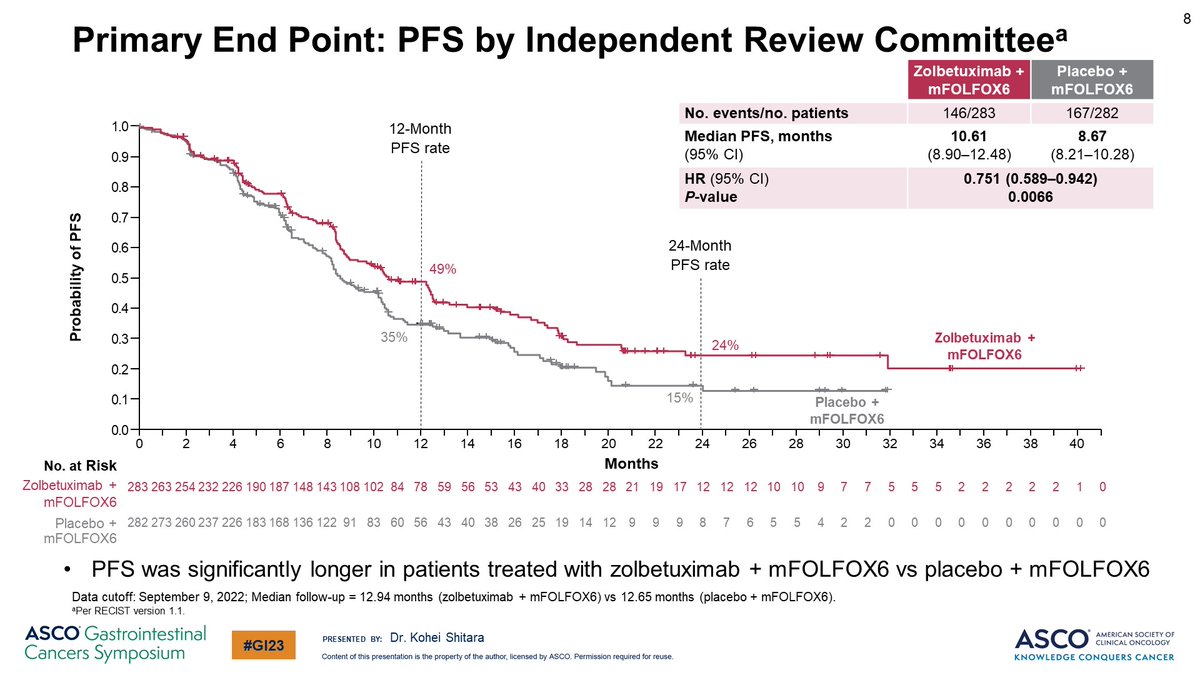
✨SPOTLIGHT
🔹median PFS: 10·61 mos (95% CI 8·90–12·48) in zolbe v 8·67 mos (8·21–10·28) in placebo
🔸Zolbe: significant reduce in death risk v placebo (HR 0·75, 95% CI 0·60–0·94; p=0·0053)
👨🏼🏫Mini tweetorial 10👨🏻🏫
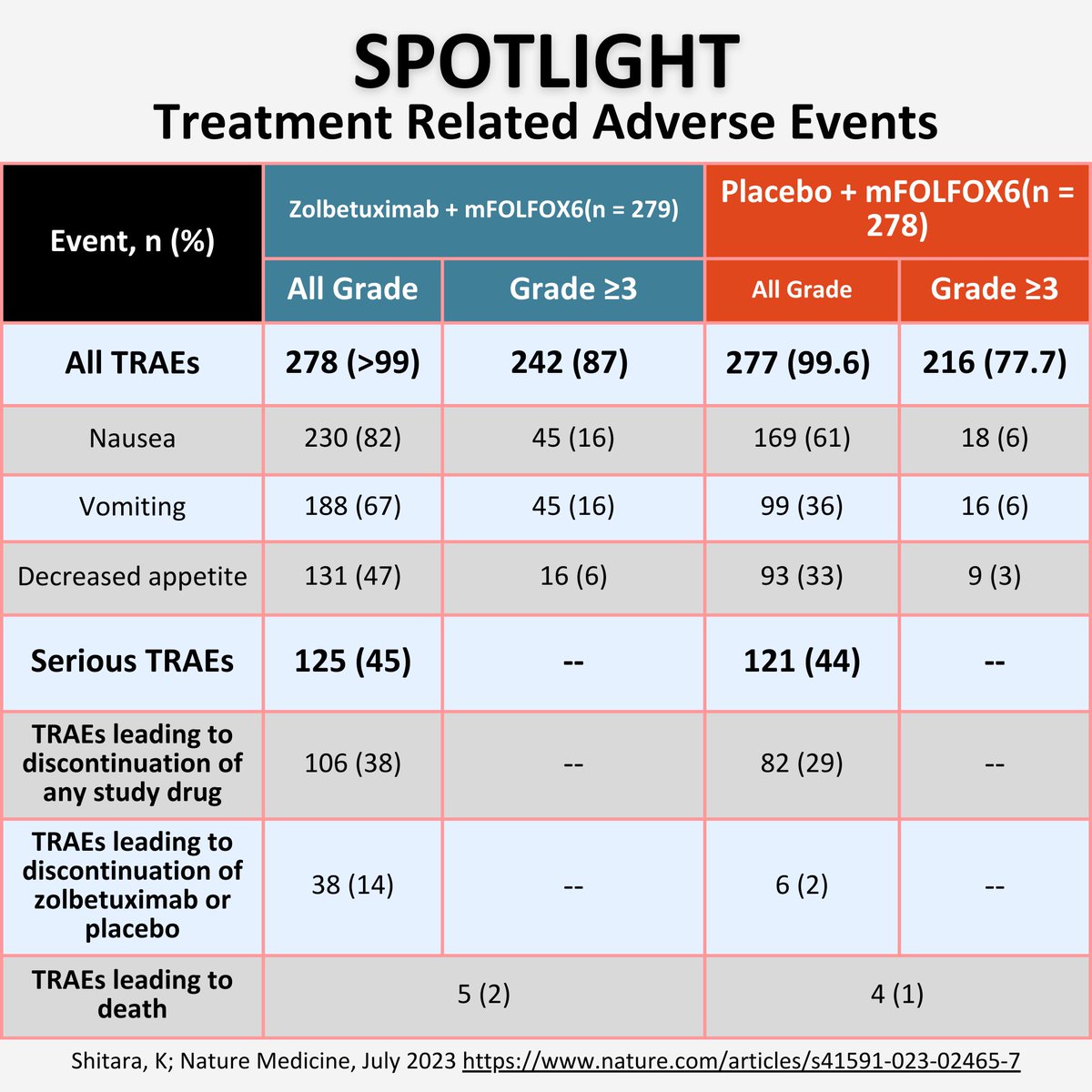
✨SPOTLIGHT
📌Safety and Tolerability of anti-CLDN18.2 mAbs
Most common AE in both studies
🤢Nausea/vomiting
🍽️anorexia
🛑TRAEs leading to drug discontinuation in 14% vs 2% in control
📚@mdmanishshah @JafferAjaniMD
👨🏼🏫Mini tweetorial 11👨🏻🏫
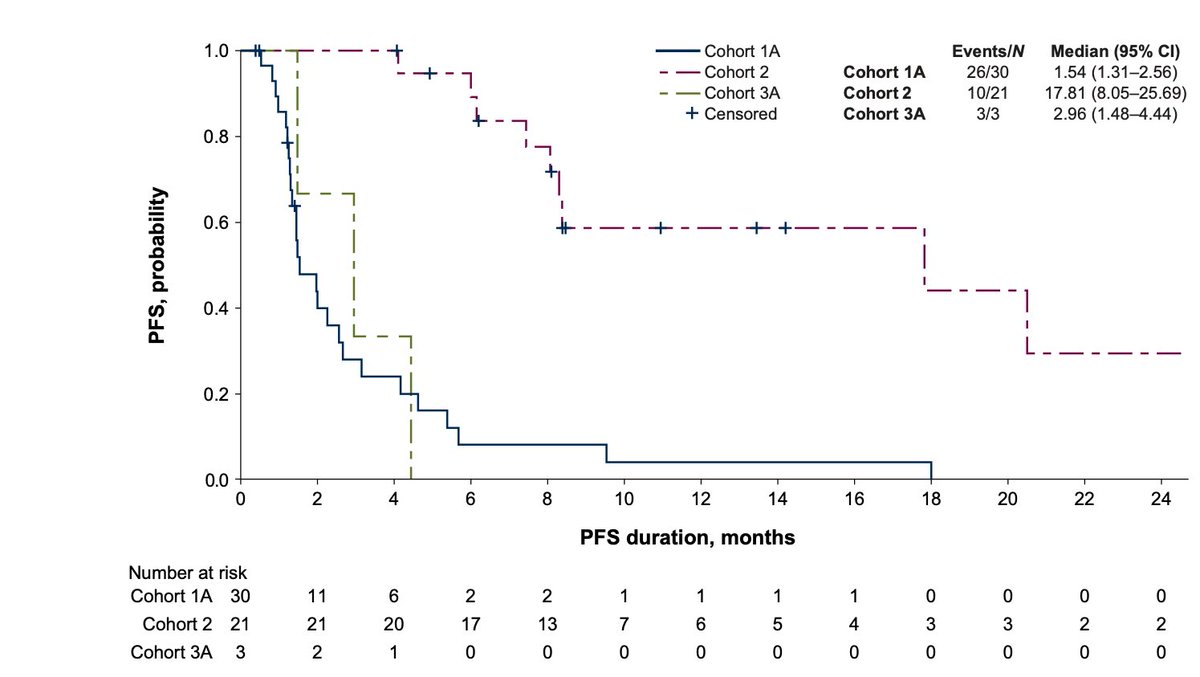
✨ILUSTRO
📌Monotx/Combo with ONLY anti-PD-1
📍Ph2
cohort 1A: monotx
🔸n=30
Median PFS: 1.54mos
cohort 3A
🔸n=3
Median PFS: 2.96mos
Both ORR: 0% vs ORR 71.4% (47-88.72): same study➡️combo with FOLFOX
mPFS: 17.8 mos
📚@KlempnerSam
Back to our case🔎
👨🏼4 cycles in study
🤢grade 2 nausea & vomiting
CEA 200 ➡️ 50
🩻CT: PR
🧪No germline or potentially actionable alterations identified on tissue NGS
💉Continued tx for 4 more cycles
🩻CT: PD
🧐What treatment would YOU suggest now?
👨🏼🏫Mini tweetorial 12👨🏻🏫
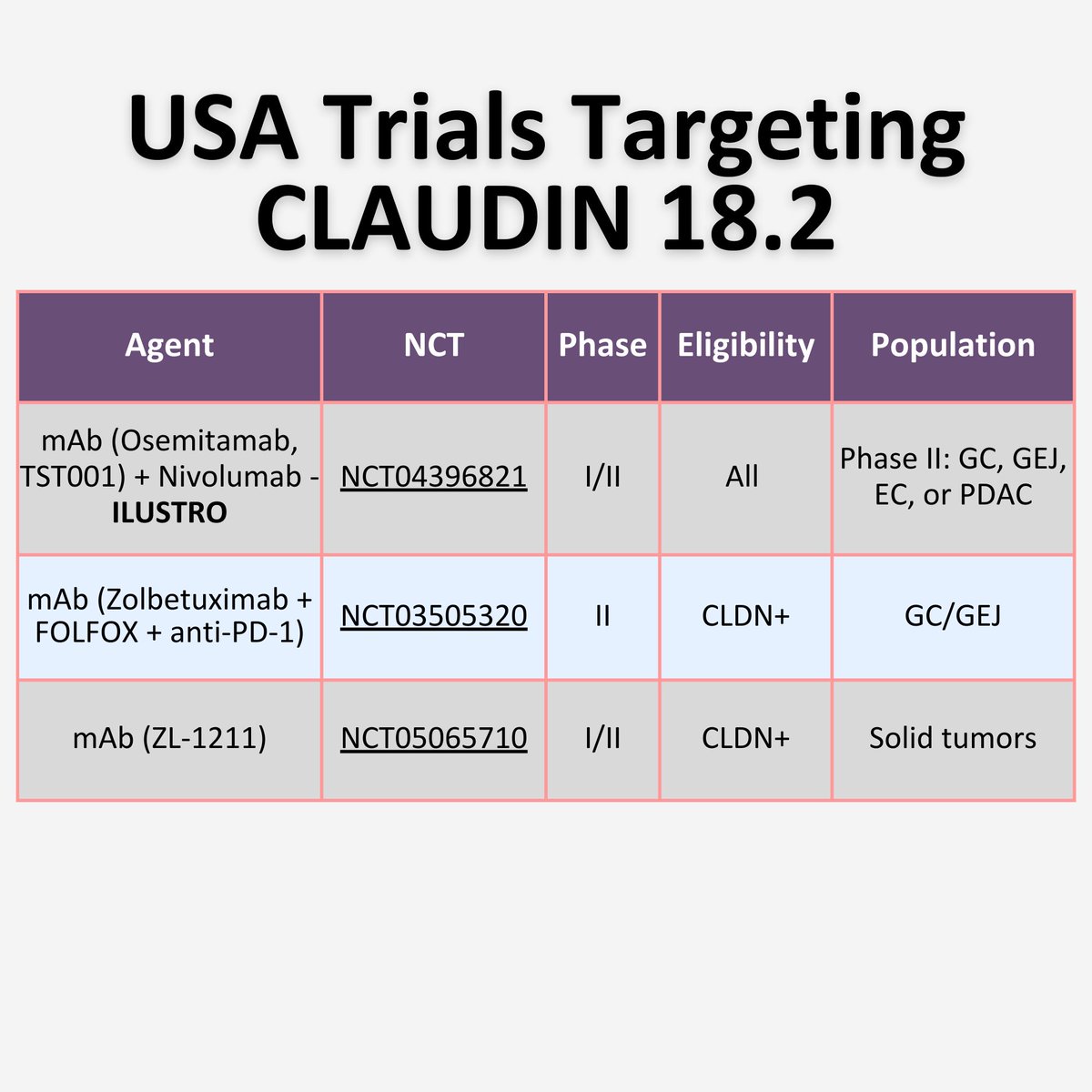
✨Trials in 🇺🇸 targeting CLDN18.2
Different therapeutic strategies currently available
Monoclonal antibody monotherapy and in combinations
🔗clinicaltrials.gov
🔗clinicaltrials.gov
🔗clinicaltrials.gov
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
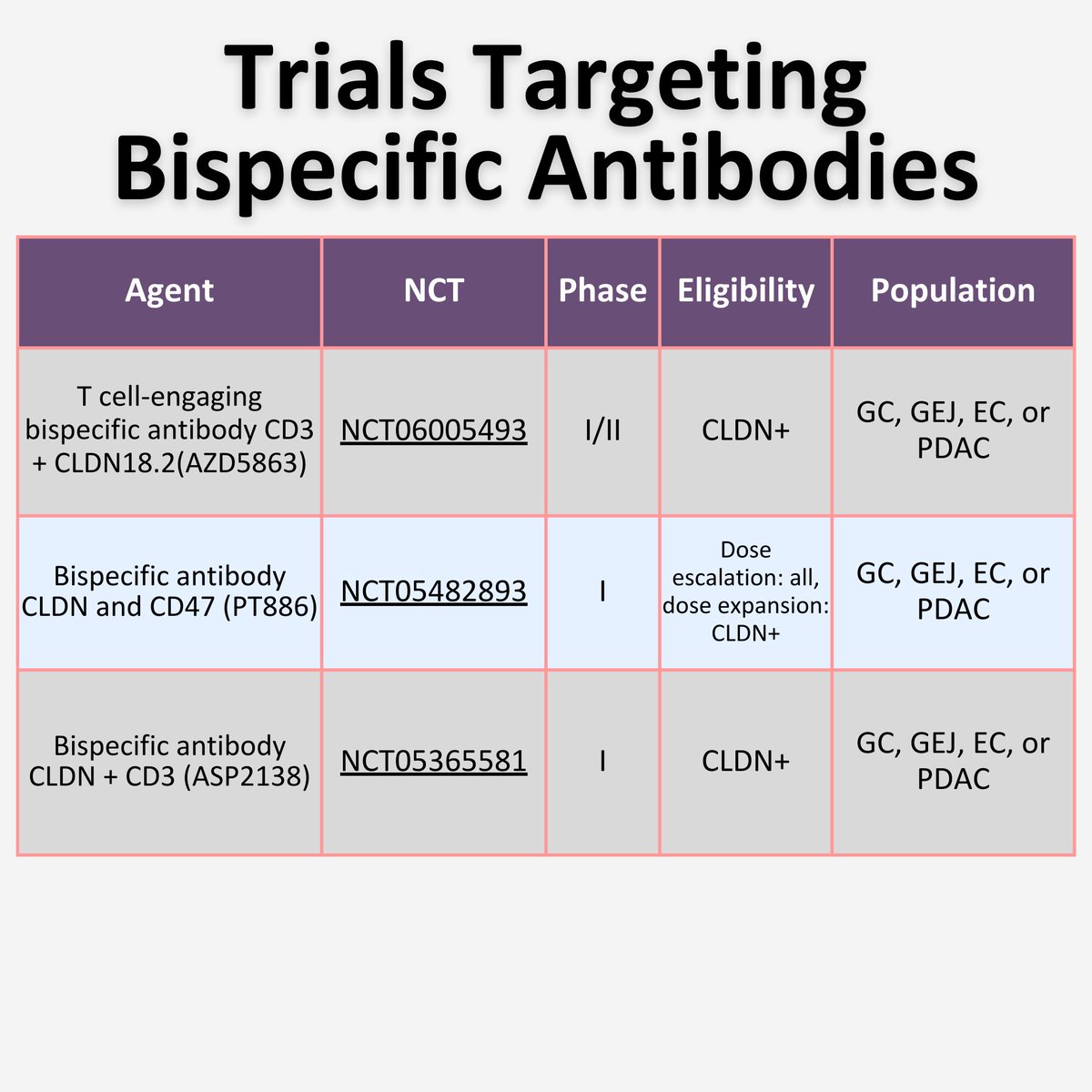
👨🏼🏫Mini tweetorial 13👨🏻🏫
📌Bispecific antibodies
2 distinct binding sites to engage T-cells with tumor cells
🧫 T-cell targets in development including CD47, CD3 and PD-L1
🔗clinicaltrials.gov
🔗clinicaltrials.gov
🔗clinicaltrials.gov
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
👨🏼🏫Mini tweetorial 14👨🏻🏫
🔠ADCs
Monoclonal antibody (mAb): linked to cytotoxic drug designed to widen therapeutic window by specific cell delivery
🔗clinicaltrials.gov
🔗clinicaltrials.gov
🔗clinicaltrials.gov
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
👨🏼🏫Mini tweetorial 15👨🏻🏫
🔠ADCs
Payload:
Topo I inhibitors, MMAE derivatives (microtubule interference), other cytotoxics, other active moieties
🔗Linker:
1° influences circulating free-drug vs release in cells
👨🏼🏫Mini tweetorial 16👨🏻🏫
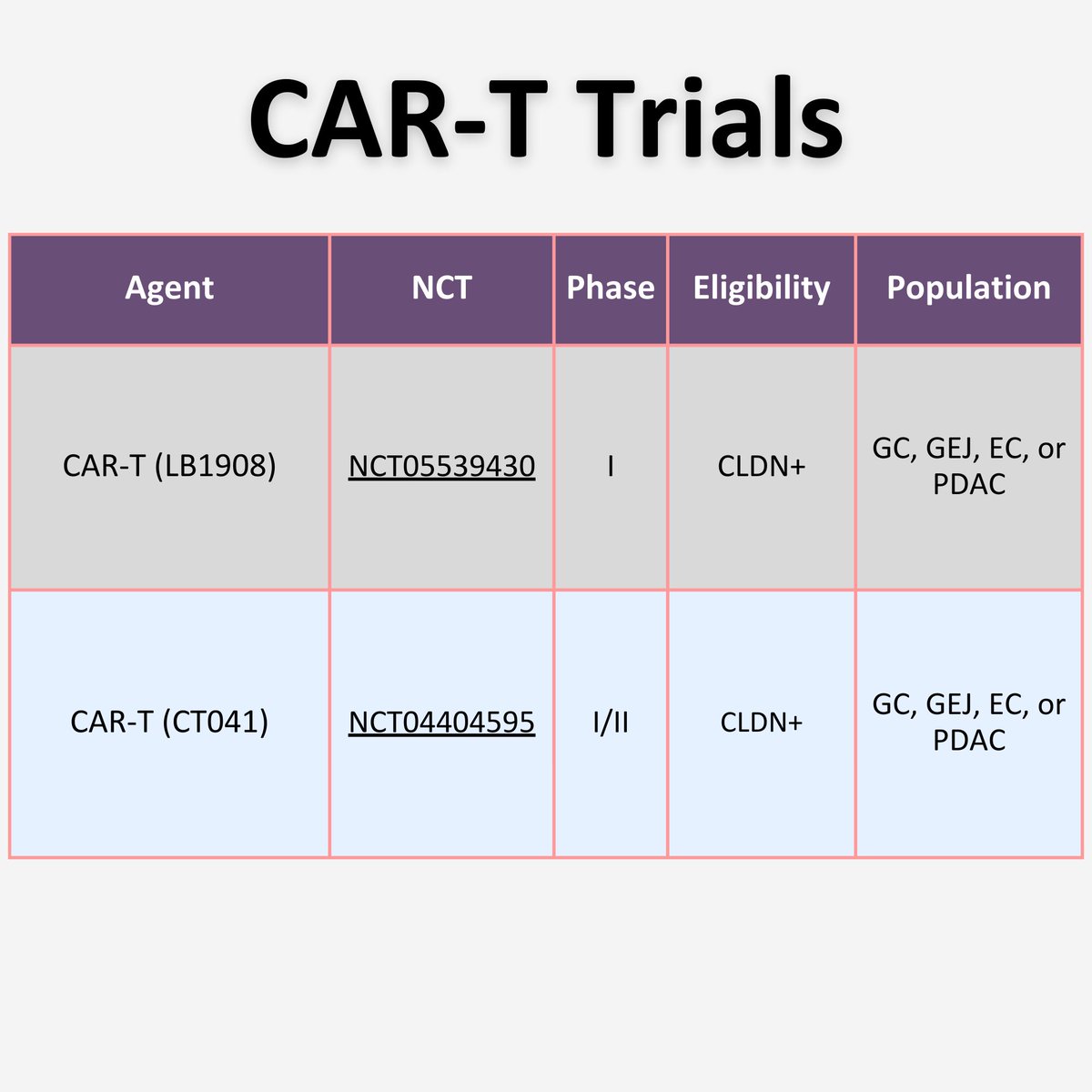
✨CAR-T
Genetically engineered autologous T cells - express CLDN18.2-targeted CAR
📍Single-arm, open-label, phase I 🇺🇸 & 🇨🇳 study
✨LB1908
🔗clinicaltrials.gov
✨CT041
🔗clinicaltrials.gov
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
CTG Labs - NCBI
Hide glossary Study record managers: refer to the Data Element Definitions if submitting registratio...
✨CAR-T CT041
CRS
🇨🇳95% (35/37)
🇺🇸93% (13/14)
all Gr 1-2
median onset 2d post-infuse
med duration 6d🇨🇳 or 2d🇺🇸
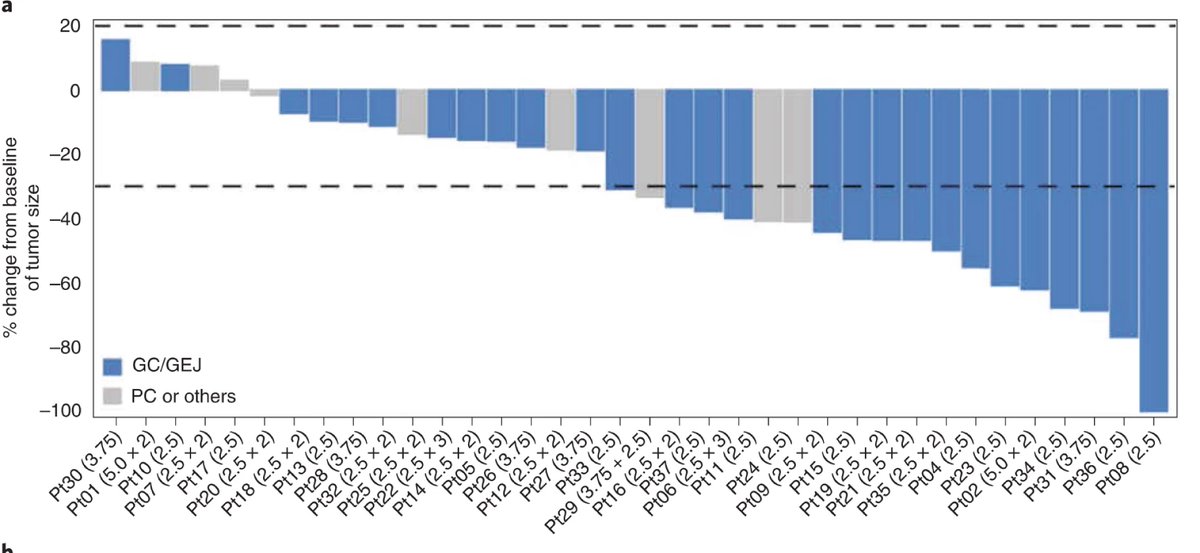
ORR in #GastricCancer
🇨🇳57% (16/28)
🇺🇸60% (3/5)
6mos DoR rate:🇨🇳53.3% (95% CI: 20.7%-77.8%)
📚@NatureMedicine
nature.com
Back to our case🔎
✂️ Rebiopsy of metastatic disease
🔬 Demonstrated persistent CLDN18.2 expression
👨🏼 Patient treated with CLDN18.2-targeted CAR with PR 🎉
🙏🏼 Disease control for 4 months
💉 Continue treatment & followup
Take home🏠
🔥New🔥 actionable target in gastro-esophageal
👍🏽Proven tx to improve PFS and OS
While awaiting✨ILUSTRO
💬Consider where in 1L tx paradigm of advanced dz
‼️UGI Cancer biomarker testing crucial‼️
🙌🏼Other strategies to🎯CLDN18.2 are on way‼️
👉🏽#CME Eval 🔗 integrityce.com
🤔@KlempnerSam @CowzerDarren taught new tx targeting CLDN 18.2, test your🧠with these Q’s
🧐What tx?
56yo👨🏽🦳
pMMR
HER2–
PD-L1+ (CPS =1)
met #GastricCancer
CLDN18.2+ (>75%) on addtl testing
*Approved or trial
👉🏽 Free CME 🔗 integrityce.com
🧐Determining CLAUDIN 18.2 status is done by which method?
جاري تحميل الاقتراحات...