👨🏻🏫Mini tweetorial 1👨🏻🏫
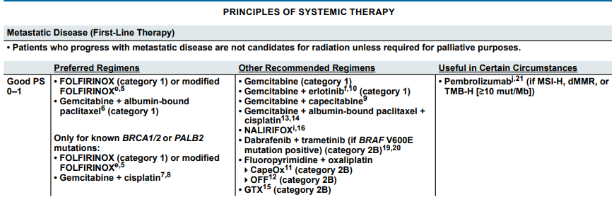
Front-line SOC systemic options for mPDAC
But
ALWAYS consider & discuss clinical trial #SharedDecision
👩🏻🦱chose to screen for ph II 1L anti-CLDN18.2 (C)
CLDN18.2 strongly (+) > 75% IHC
👩🏻🦱randomized into study: receive C + Gem/Nab-P
👨🏻🏫Mini tweetorial 2👨🏻🏫
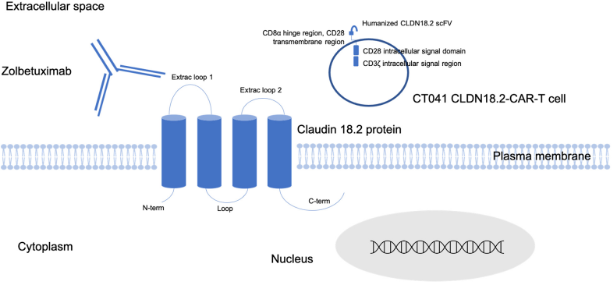
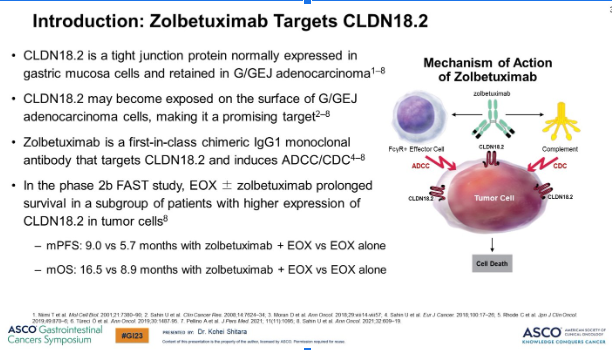
📌What is CLAUDIN 18.2
📍cell-to-cell tight junction protein
📍major role in cell polarity & barrier function
📍Alternative splicing variants at 1st exon of Ch’some 3q22
📚@giuliagrizz
👨🏻🏫Mini tweetorial 3👨🏻🏫
📌CLDN18.2
📍expresses as cancerous tissue in subgroups of
🔹Pancreas
🔸Gastric
🕵🏻being explored in others (biliary tract & lung)
⬆️highly expressed can be attractive drug delivery 🎯 or immunotherapy
👨🏻🏫Mini tweetorial 4👨🏻🏫
📌Anti-CLDN 18.2
🔦SPOTLIGHT: Ph III of FOLFOX + Zolbe/Placebo
📚@KoheiShitara @ILSONDavid @JoeChaoMD @JafferAjaniMD t.ly
🕯️GLOW: Ph III CAPOX + Zolbe/Placebo
📚@mdmanishshah t.ly

Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial
Targeting CLDN18.2 with zolbetuximab significantly prolonged progression-free survival and overall s...

Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial
Nature Medicine - In the randomized, double-blind, phase 3 GLOW trial, capecitabine and oxaliplatin...
👨🏻🏫Mini tweetorial 5
📌Ph III studies in gastric ca: mFOLFOX +/- Zolbetuximab
🔦SPOTLIGHT
🗝️1° endpoint PFS met mPFS of 10.6 for zolbe + mFOLFOX6 vs 8.7 for mFOLFOX6, HR 0.75, p=0.0066
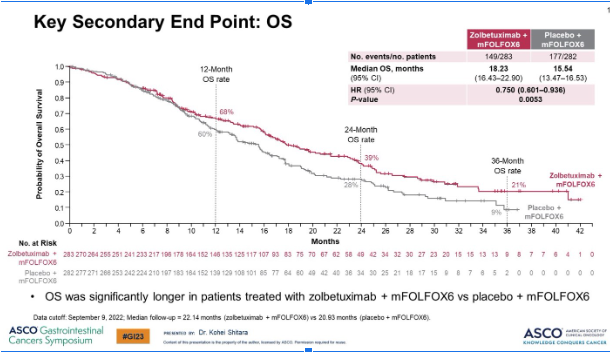
🔦2° endpoint OS met mOS 18.2 for zolbe + mFOLFOX6 vs 15.5 for mFOLFOX6
👨🏻🏫Mini tweetorial 6👨🏻🏫
🔦SPOTLIGHT
🎯1st molecular target Tx with 1L OS benefit of aGC since Herceptin
📍87% w PD-L1 CPS<5% (familiar in panc?)
📍Ctrl did well
👏🏼Study arm: major survival improvement!
🧐⬆️ OS for ⬆️CLDN18.2 expression?
👨🏻🏫Mini tweetorial 7👨🏻🏫
📍Large study #AACR23 reviewed different CLDN18.2 levels & OS
📍Differing CLDN18.2 levels at 50% & 75% showed no OS difference.
📚@JafferAjaniMD t.ly
👨🏻🏫Mini tweetorial 8👨🏻🏫
📌Phase III study of chemo + Zolbe
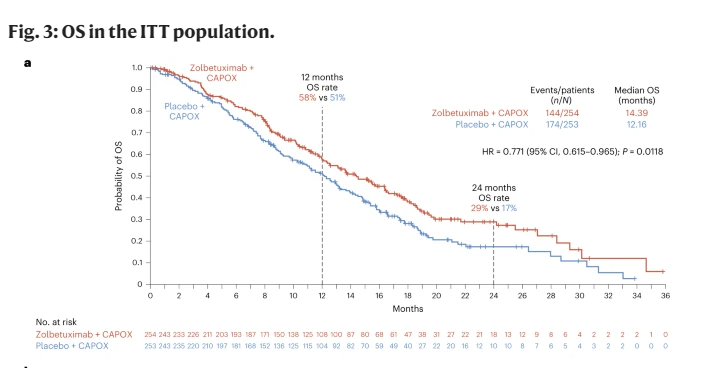
🕯️GLOW
🔹N=507
💉CAPOX vs CAPOX + Zolbe
📈mPFS 8.21m CAPOX + Zolbe vs 6.80 CAPOX
📉mOS 14.39 CAPOX + Zolbe vs 12.16 CAPOX
📚@mdmanishshah t.ly
👨🏻🏫Mini tweetorial 9👨🏻🏫
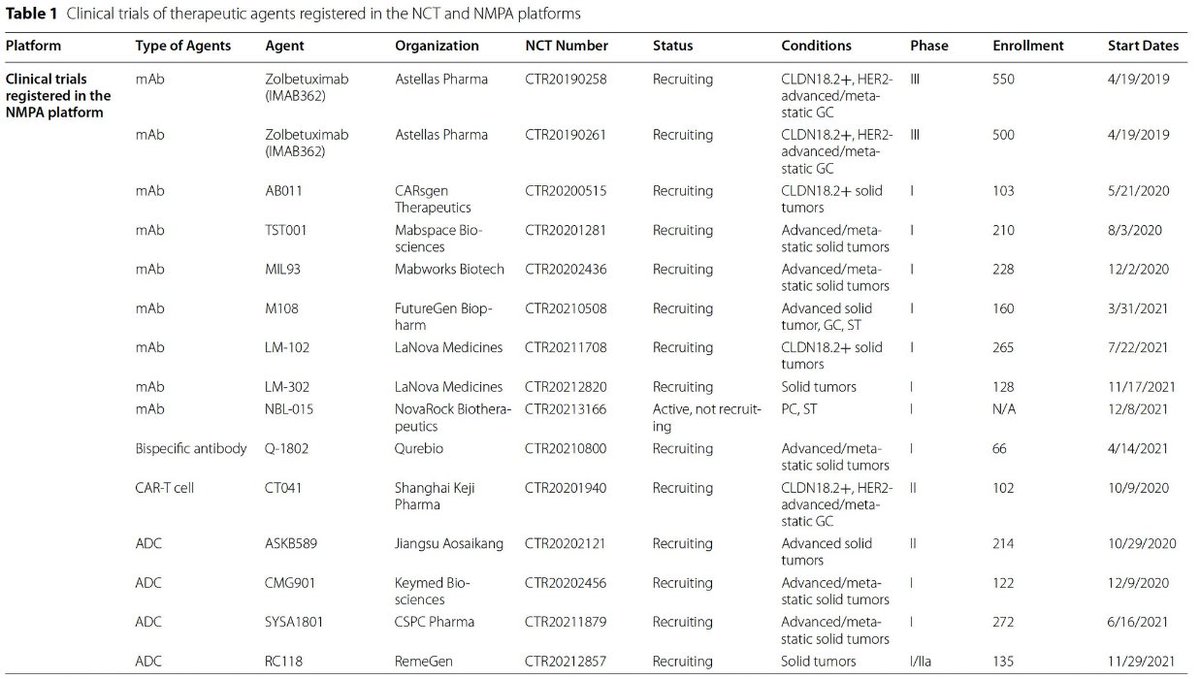
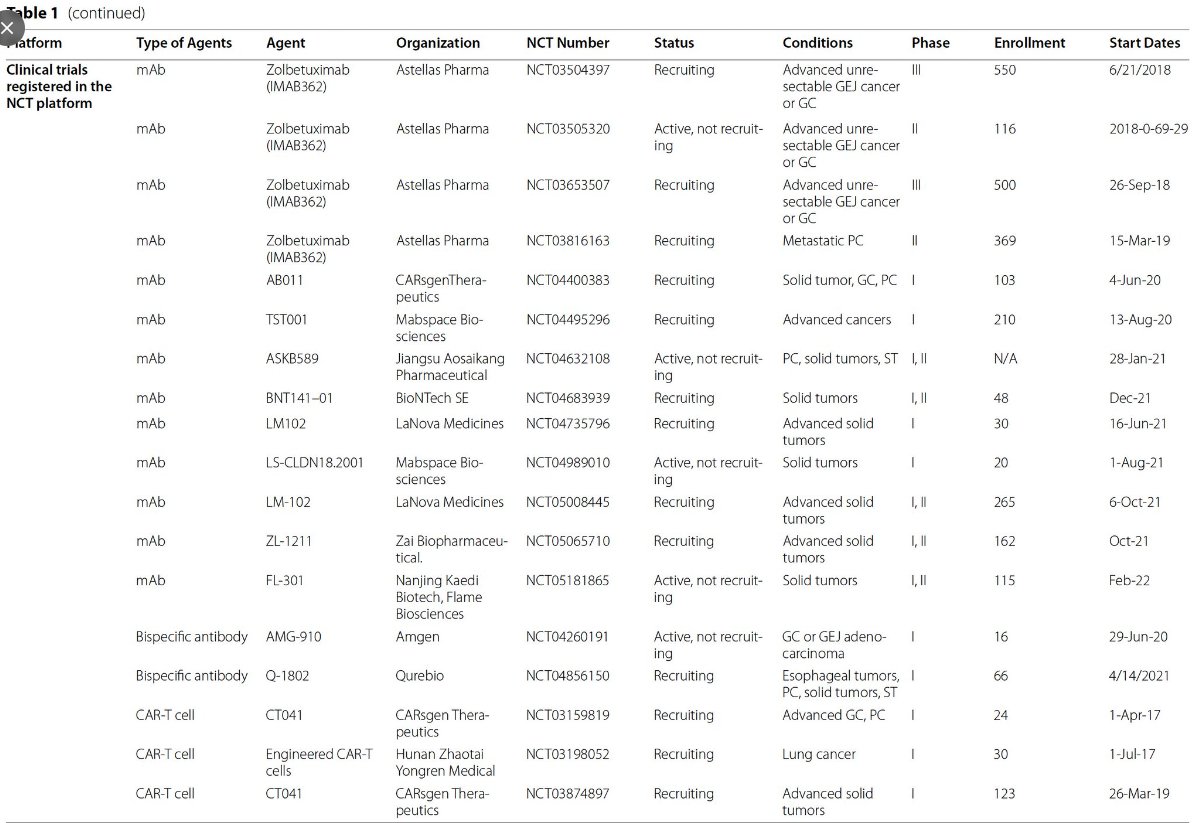
Lots of Zolbe data for CLDN in GC/GEJ, what about #PancreaticCancer?🤔
‼️Zolbetuximab trial in mPDAC recruiting‼️
📚tinyurl.com
🎲IHC >75% can be randomized in 2:1 for GN+Z or GN-Z
Expected accrual ‘24
👀Stay tuned‼️
Back to case🔎
💉After 1st infusion
🤢significant nausea with GN antiemetics (dexameth or 5HT3 antagonist)
🍽️reduced appetite
⬇️weight
Given 👩🏻🦱 severe nausea impacting multi facets, considering stopping trial
What do we know about Zolbe 🤢 + anorexia?
👨🏻🏫Mini tweetorial 10👨🏻🏫
🔦SPOTLIGHT
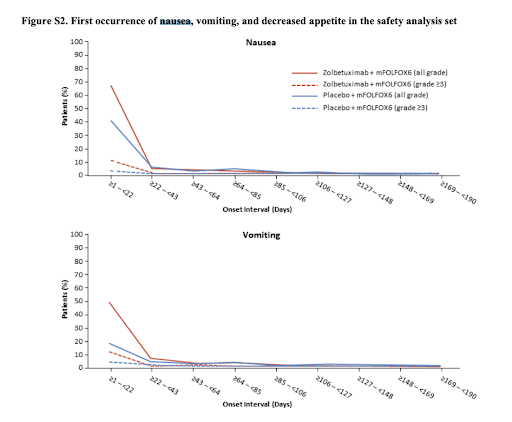
🤢Nausea/Vomiting
📍Common at C1 but G3 rare
📍All G 82% in Zolbe arm vs 61% in Placebo arm
📍> G3 16% in Zolbe arm vs 6% in placebo arm
📍Discontinue: 6% in ZOL arm vs 1% in placebo arm
👨🏻🏫Mini tweetorial 11👨🏻🏫
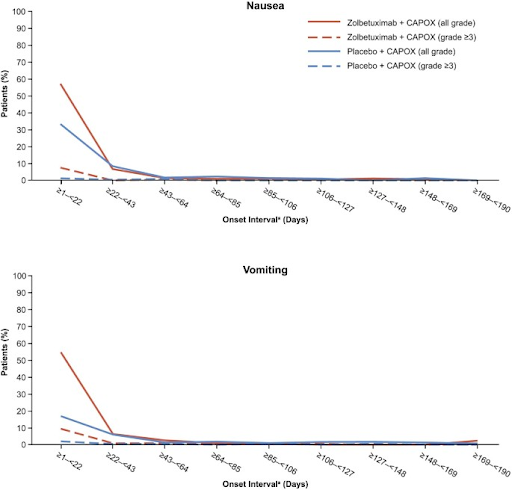
🕯️GLOW
🤢Nausea/Vomiting similar to 🔦SPOTLIGHT
🗝️Early anti-emetics per institution standard
🚰Supportive tx
🥛Consider supplements/dietitian
👨🏻🏫Discuss with pt & family 🤢 improves after 1-2 C
Back to case🔎
🙌Nausea & anorexia👌🏽2 cycles
🩻CT: PR
🩸CA 19-9: 300
🧪Genomic results:
Germline (-)
Somatic: MSS< TMB 3, KRAS G12C, TP53
👩🏻🦱Continuing trial
🗓️Stable for 4 scans
BUT
😖Back pain
⬆️CA 19-9
🩻CT: dz progress➡️liver & pancreas
🤨Now what?
👨🏻🏫Mini tweetorial 12
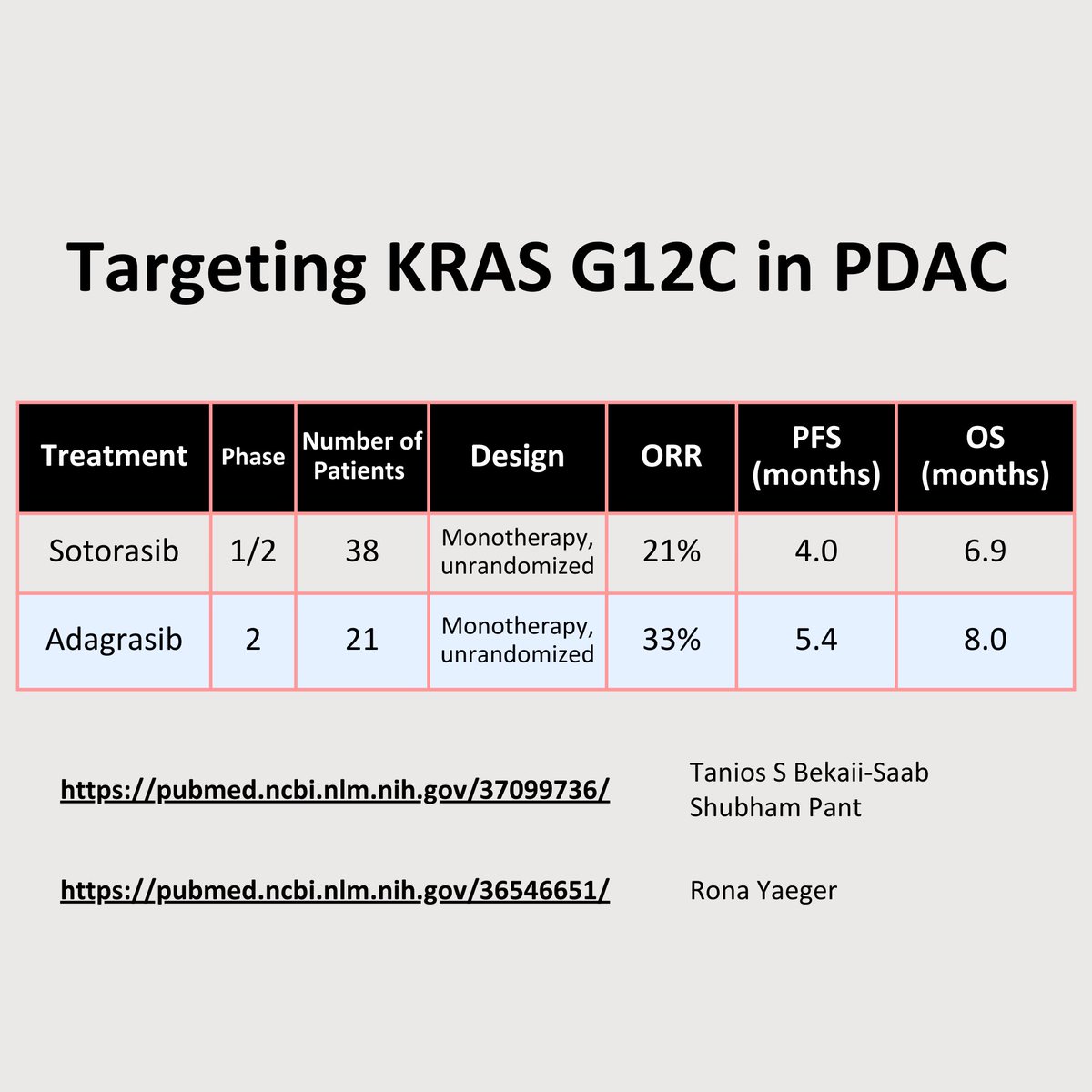
🎯KRAS G12C in PDAC
G12C rare in PDAC: 1-2%
#KRAS trial results😍 adagrasib, sotorasib, divarasib, + more developing
🕵🏻other, non-G12C muts
📚@GIcancerDoc @DrShubhamPant pubmed.ncbi.nlm.nih.gov
📚@RonaYaeger pubmed.ncbi.nlm.nih.gov

Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer - PubMed
Sotorasib showed anticancer activity and had an acceptable safety profile in patients with KRAS p.G1...

Adagrasib in Advanced Solid Tumors Harboring a KRASG12C Mutation - PubMed
Adagrasib demonstrates encouraging clinical activity and is well tolerated in this rare cohort of pr...
We’ve discussed PDAC assays
🧪CLAUDIN 18.2
🧪NGS including *KRAS G12C strategies
*Many other KRAS inhibitors coming in next tweets!
BUT first
Looking back to tweet 5,
🧐What do we know about CLDN18.2 in PDAC?
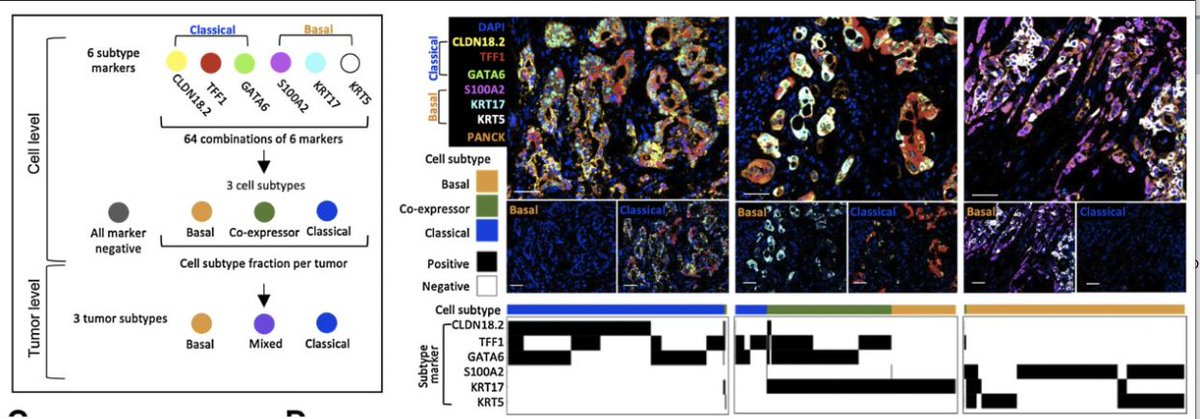
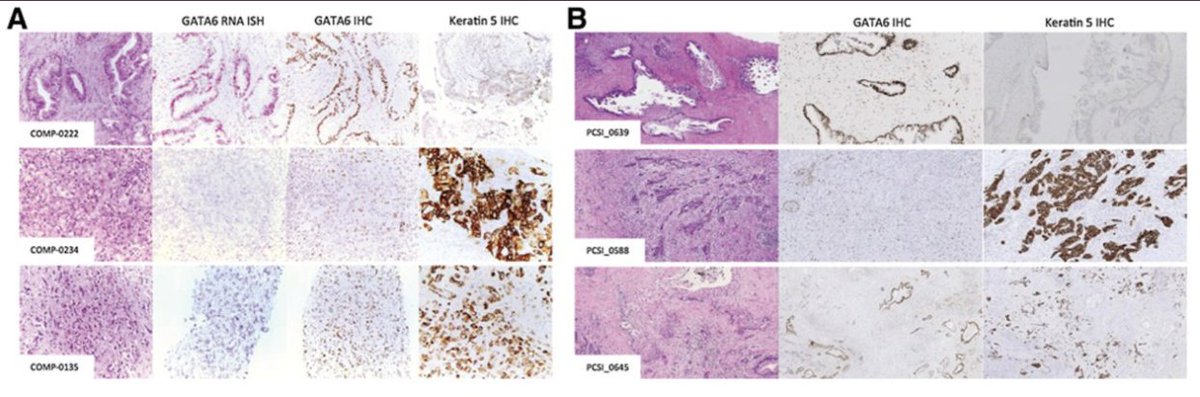
Transcriptomic subgroups define diff prognosis in #PancreaticCancer
🕵🏻Using RNAseq, prospective evaluation of these subgroups with SOC chemo
✨COMPASS ✨PASS01
📚@graokane pubmed.ncbi.nlm.nih.gov
📚 pubmed.ncbi.nlm.nih.gov

GATA6 Expression Distinguishes Classical and Basal-like Subtypes in Advanced Pancreatic Cancer - PubMed
The basal-like subtype is chemoresistant and can be distinguished from classical PDAC by GATA6 expre...

Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial - PubMed
Purpose: To perform real-time whole genome sequencing (WGS) and RNA sequencing (RNASeq) of advanced...
We got carried away~
Back to our case 🔎
👩🏻🦱Pt decided CLDN18.2-ADC
🎉thriving & discussing other anti-CLDN18.2 strategies🙏🏼
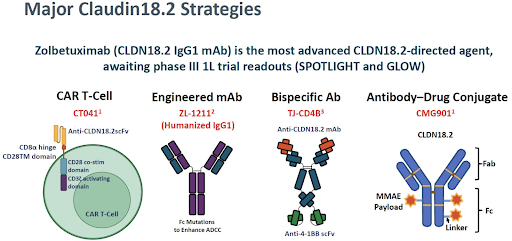
Other pending strategies under investigation:
📚Shen #ASCO23 diff anti-CLDN18.2 mAb
📚Cao. Biomarker Res 2021, Konno #AACR21, Jiang #AACR20
biomarkerres.biomedcentral.com
💉mRNA vaccine
fiercebiotech.com
@MaenAbdelrahim

Claudin18.2 is a novel molecular biomarker for tumor-targeted immunotherapy - Biomarker Research
The claudin18.2 (CLDN18.2) protein, an isoform of claudin18, a member of the tight junction protein...

Moderna reveals Claudin18.2 ambitions via cancer vaccine, solid tumor CAR-T combo plans
What could be hotter than Moderna getting in the Claudin18.2 race? How about pairing up a newly disc...
Take🏡 points
PDAC needs comprehensive test for many biomarkers‼️
CLDN18.2 is a new defined biomarker for GI cancers, various strategies‼️
Zolbe trial recruiting‼️
👉🏽#CME Eval 🔗 integrityce.com
👉🏽ALL CME🔗 integrityce.com
🤔@CentralParkWMD @ferguskeane2 taught us CLDN18.2 in #PanCan test your 🧠 with quick❓
🧐Which testing would you use to determine a patient's CLAUDIN 18.2 status?

Tumor Board Tuesday 2023 Conversations
Tumor Board Tuesdays is a regularly scheduled, Twitter-based case discussion forum led by expert fac...

Tumor Board Tuesday Evaluation 2023 (ID: i854/i855/i862/i865)
Take this survey powered by surveymonkey.com. Create your own surveys for free.
👉🏽 Free CME 🔗 integrityce.com
🧐What regimen* would you select?
58yo 👩🏼🦳
metastatic pancreatic ductal adenocarcinoma
CA 19-9: 680
CLDN18.2: 86%
*approved OR investigational
Loading suggestions...