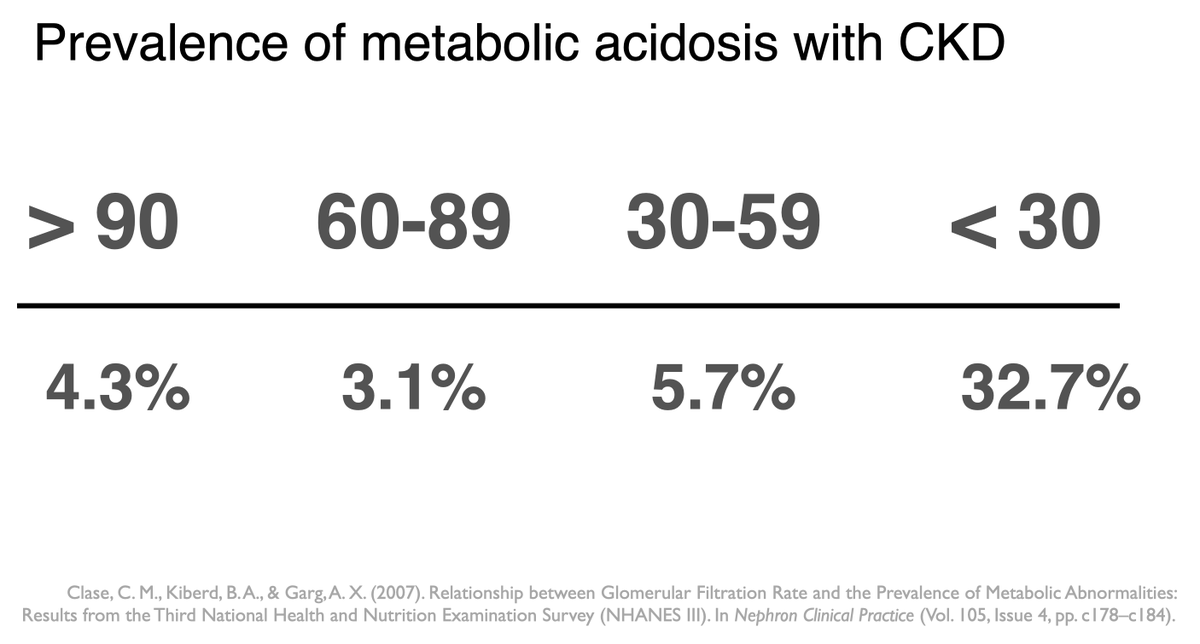My talk at #ApExPathshala on #AcidBase had some new (to me information) on urine anion gap. I want to share it but to get there we need to discuss renal acid handling. #Tweetorial #Physiology #Electrolytes 1/11
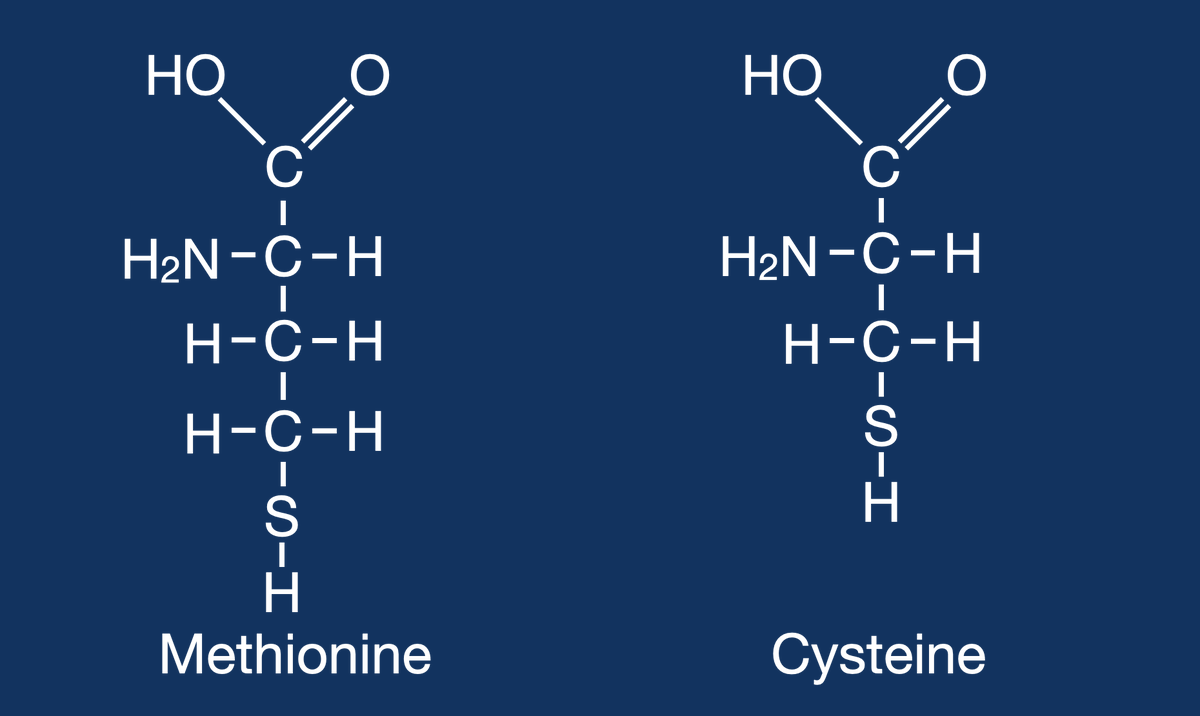
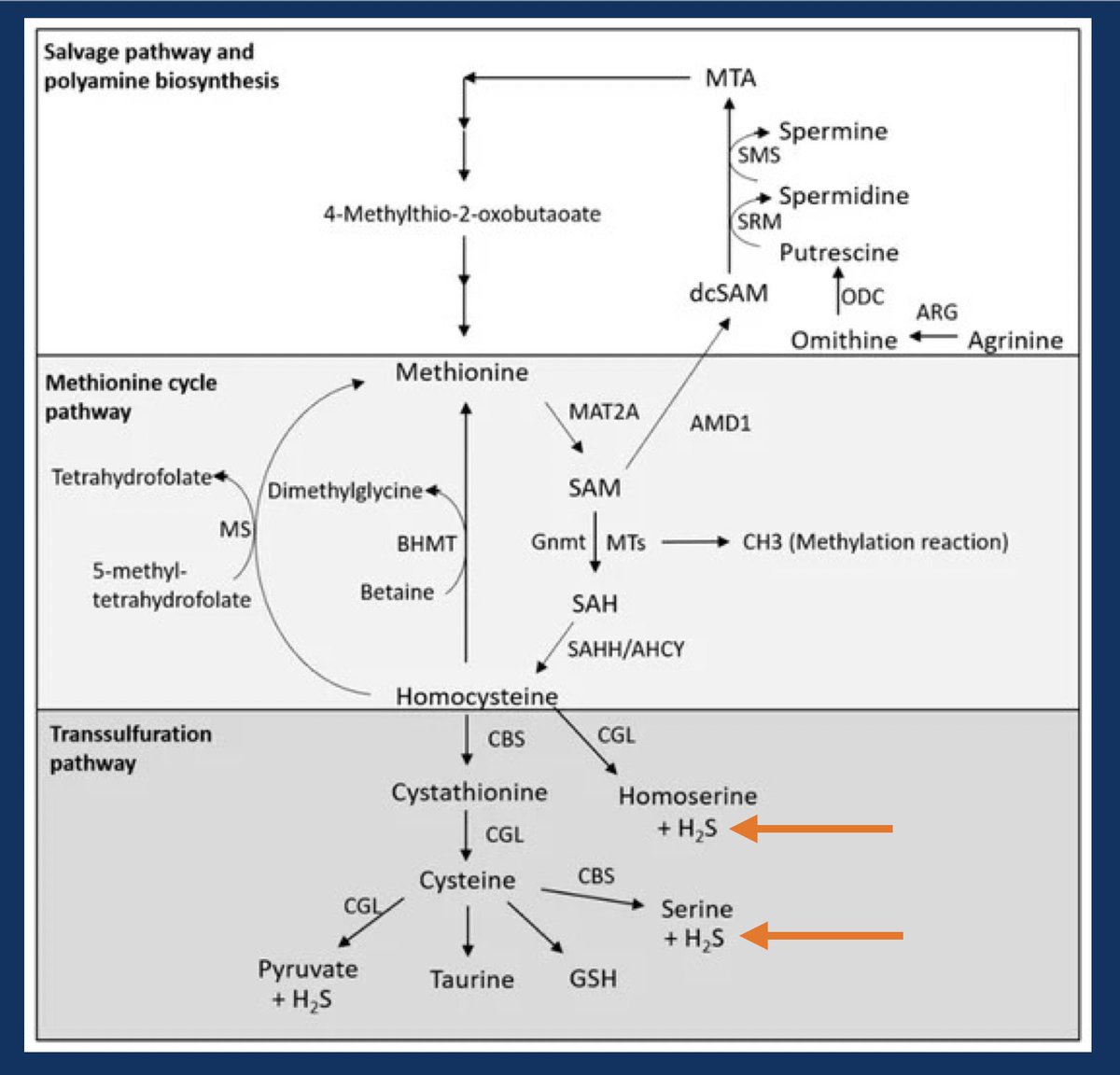
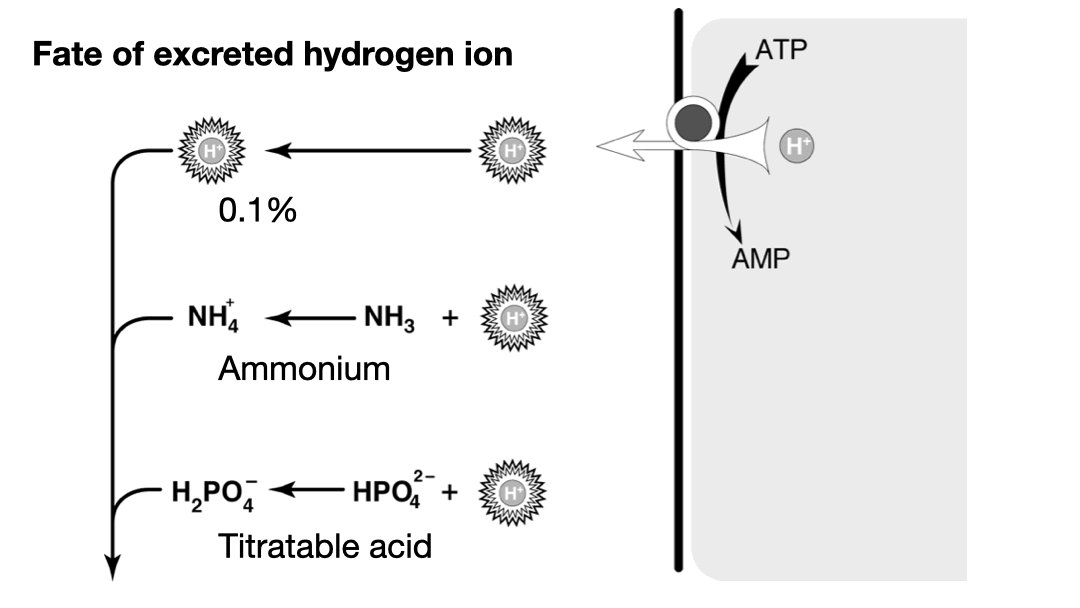
Metabolizing proteins, specifically the sulfur containing amino acids, methionine and cysteine, generates hydrogen sulfide (H2S). This acid cannot be cleared by the lungs. #OnlyTheKidneys can clear this acid. 2/11
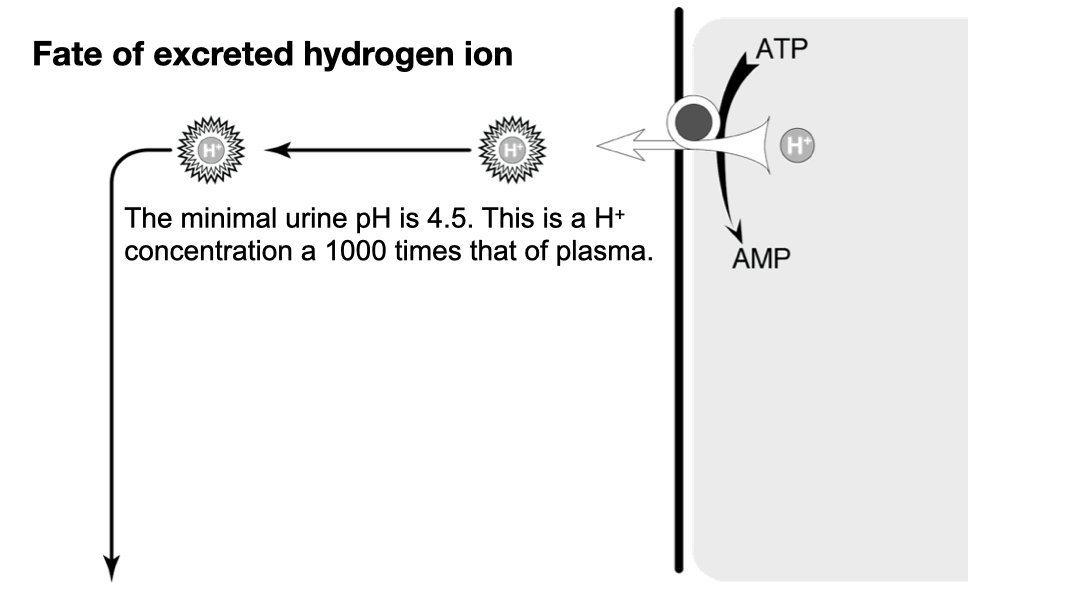
On a “western diet” (I.e. carnivorous diet) it is about 50-100 mEq of acid (H+ ions) a day. 4/11
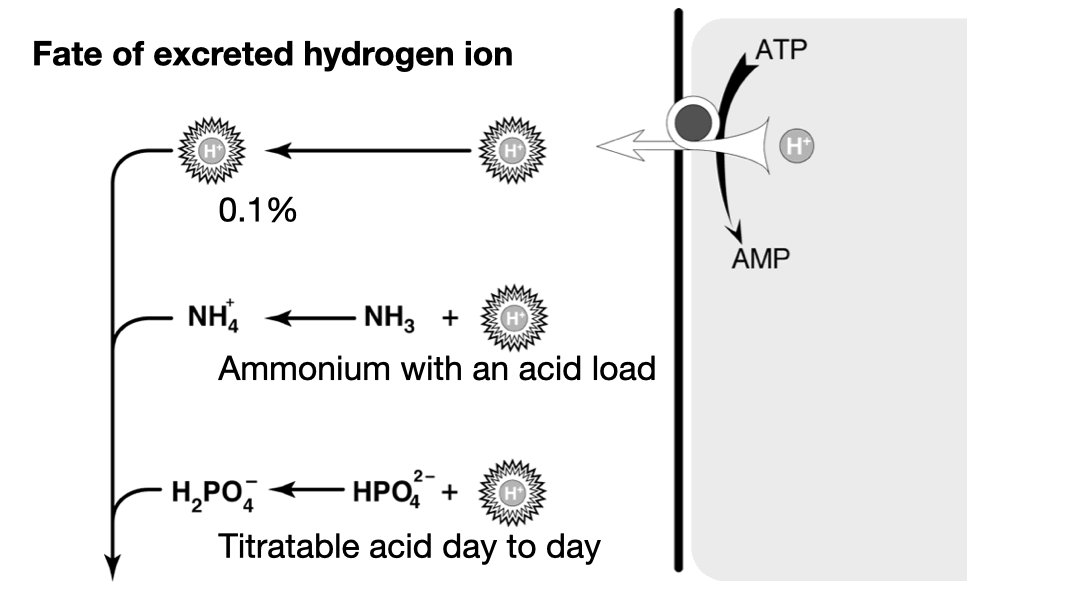
Titratable acid is just H2PO4–. Most of the daily acid load is excreted this way. The problem is that it is fixed by phosphate intake. We cannot manufacture new phosphate when we encounter a large acid load, so we cannot ramp up titratable acid to deal with an acid load.** 7/11
**Actually that is not entirely true. In addition to the serum bicarbonate, the bones are called upon to buffer an acid load. And as they are dissolved buffering acid (not an ideal state) they release phosphate which clears the acid from the body. pubmed.ncbi.nlm.nih.gov 8/11
So when faced with a large acid load we call on system two: ammonium. The physiologists have two models for how this works. In one the production of NH4 from glycine produces two bicarbonate, the other urinary NH3 accepts a hydrogen ion to form NH4. 9/11
We’ll let the physiologists argue over these two models, for our purpose the only thing you need to know is that a healthy renal response to acidosis is an increase in urinary ammonium to excrete the excess daily acid load. 10/11
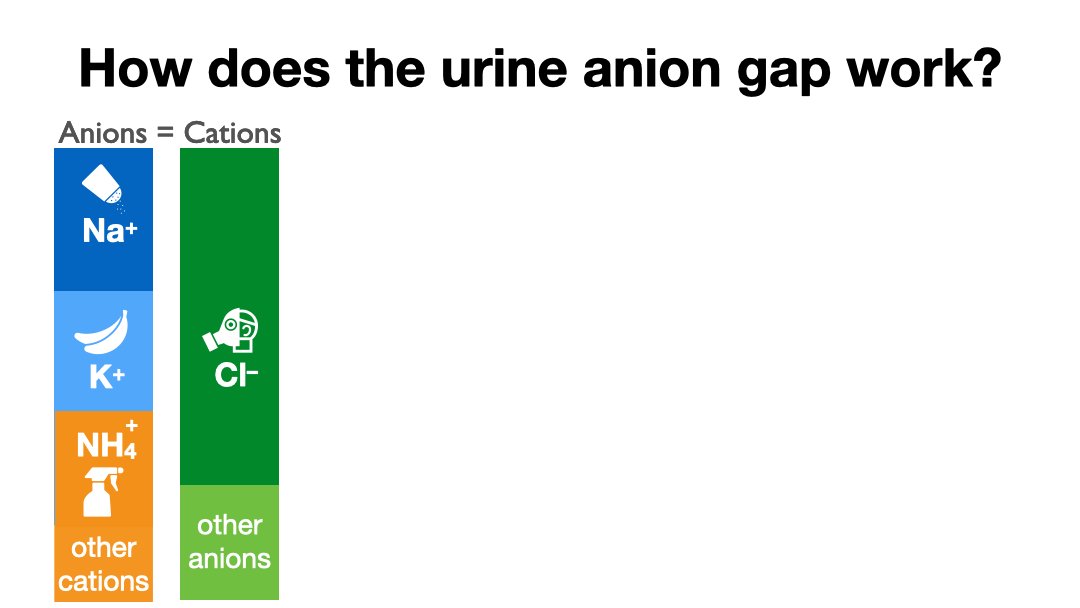
The problem comes from the fact that when you order a urine ammonium the lab tells you to pound sand. Even though they can use the same instrument as for serum ammonia. Because the lab won't measure urine NH4 we have substituted the anion gap.
To be continued...
11/11
To be continued...
11/11
Okay, before we move on to part two, let’s review,
The kidneys excrete 50-100 mEq of H+ ions every day, “The daily acid load”
1/15
The kidneys excrete 50-100 mEq of H+ ions every day, “The daily acid load”
1/15
Clinical labs generally refuse to measure urinary ammonium so doctors have been forced to scramble to find ways to “estimate” urinary ammonium.
3/15
3/15
So for forty years this schema ruled the nephrology wards.
Patients with non-anion gap metabolic acidosis would have urine electrolytes checked in order to see if their kidneys were responding appropriately
7/15
Patients with non-anion gap metabolic acidosis would have urine electrolytes checked in order to see if their kidneys were responding appropriately
7/15
Urine sodium equals dietary sodium. The same goes for potassium and chloride. Spot samples may vary for a short period of time but eventually must reflect dietary intake.
10/15
10/15
Additionally, DKA, respiratory acidosis, and systemic acidification all result in increased urine ammonium without making the urine anion gap more negative. (refs here: pbfluids.com)
13/15
13/15
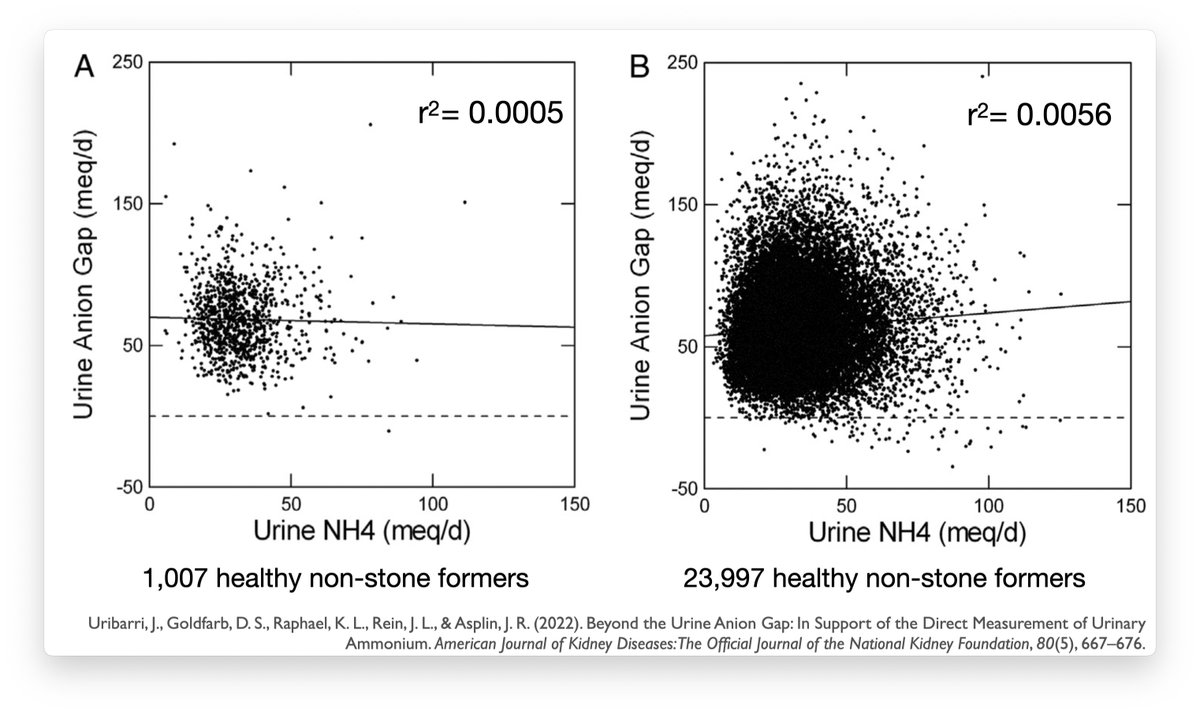
Summary: urinary anion gap is not a good measure of urine ammonium and should be abandoned.
If you need to know the urinary ammonium, measure the urinary ammonium.
15/15
If you need to know the urinary ammonium, measure the urinary ammonium.
15/15
Loading suggestions...